To date, there are no known reports of this condition occurring secondary to drugs. Here, we describe, with interest, a novel case of pseudobulbar affect occurring in a specific pattern in the context of chemotherapy use.
Case Report
In August 2011, a 52-year-old Chinese woman, nonsmoker, nondrinker, divorced, with an 18-year-old daughter, without any family history of mental illness or significant past psychiatric history, was admitted to our hospital for the initiation of a course of chemotherapy.
In 2006, she was diagnosed with infiltrative ductal carcinoma of the right breast, which was treated with right-sided wide excision and Level 2 axillary clearance. She completed 6 cycles of chemotherapy, at the time uneventfully, with a regimen consisting of doxorubicin, cyclophosphamide, and 5-fluorouracil. She had been given intravenous dexamethasone before every cycle of chemotherapy at the time to reduce acute emesis.
2 Thereafter, she also completed 2 years of tamoxifen therapy.
In June 2011, she was diagnosed with invasive ductal carcinoma of her left breast, which was treated with left total mastectomy and sentinel node biopsy. She was planned for a course of 6 cycles of chemotherapy, each cycle consisting of carboplatin, docetaxel, and trastuzumab, followed by a short course of filgastrim.
Upon admission, she underwent her first cycle of chemotherapy uneventfully. However, she was then referred to the Consultation–Liaison Psychiatry team post-chemotherapy, presenting with episodic emotional lability (interspersed laughing and crying spells), associated with low mood and anxiety.
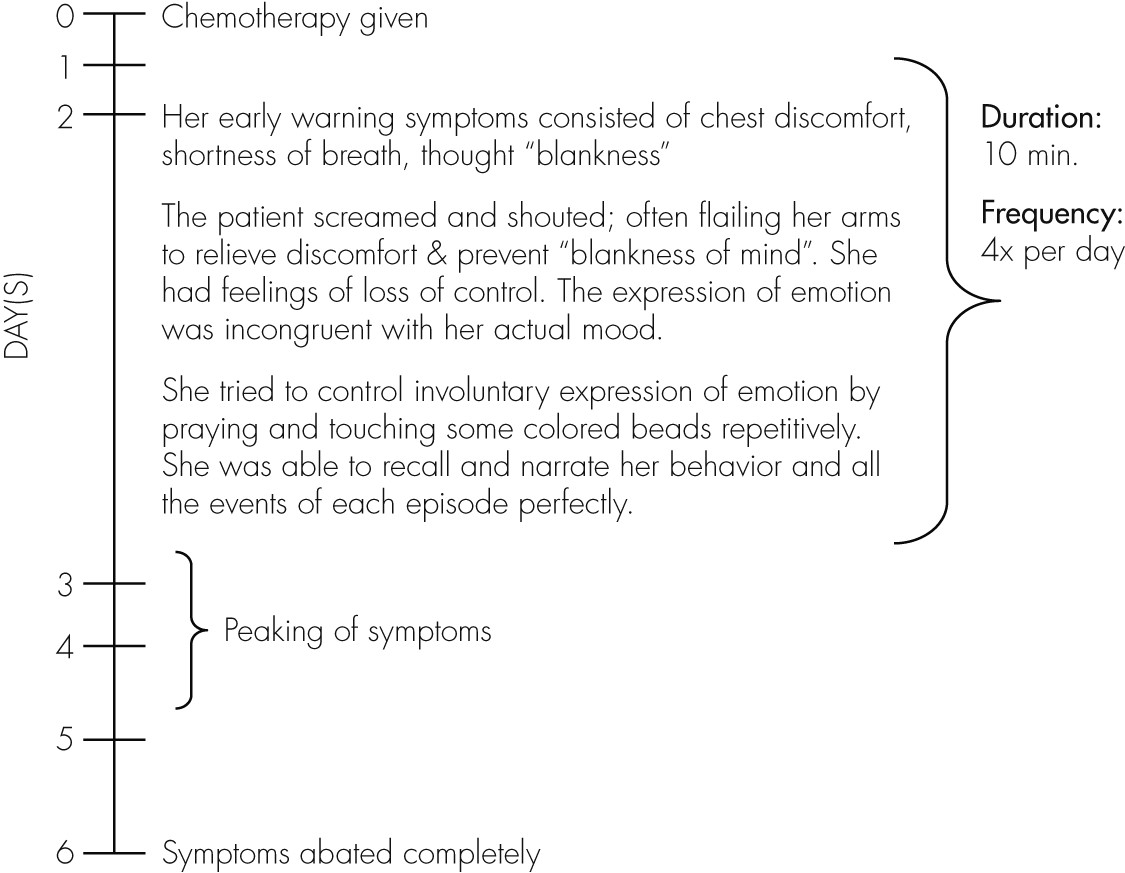
History from the patient, corroborated by her family members, revealed a specific pattern associated with her symptoms (
Figure 1).
Her mood subsequently became low after the onset of her presentation, partly due to her lack of understanding of the cause, but mainly because her family believed that her symptoms were due to depression and denial of her illness. However, the patient did not have any preceding pervasive symptoms of depression. There was no history of manic symptoms.
Indeed, she expressed her immense motivation to live and survive the cancer for the sake of her daughter, to whom she was very close. Despite her increasing anxiety and fear of the chemotherapy cycles, she was determined to complete the designated course.
Family and Personal History
She was the 4th of 10 children. Her relationship with her siblings was close. She had good family support. She was known to be an active and independent woman, who was easily anxious and stressed. She coped with stress by confiding in her family, and this has stood her in good stead over many hurdles in her life. There was no significant substance or forensic history. She has no other significant medical history, other than that described above. She had no history of childhood seizures.
Mental State Examination
This was a thin Chinese lady, with sparse, graying hair and neat appearance. She had good eye contact and was cooperative with the interview. Rapport was easily established.
Her mood was described to be normal, and her affect was reactive at the commencement of the interview. Her speech was relevant and coherent. There was no pressure of speech nor was there rapid shifting of topic-to-topic.
Midway through the interview, she abruptly stopped speaking. There was unprovoked laughter, interspersed with crying spells, lasting for about 5 minutes. Immediately afterward, she murmured prayers to herself quietly while grasping a colored bead bracelet. She completely ignored my queries or calls for an additional 5 minutes. Afterwards, she was able to narrate accurately back to me the questions I asked during this interim. She had been aware of my queries, but she would not allow herself to answer at that point for fear of relapse of the episode.
There were no psychotic features elicited. She was not thought-disordered. Her short-term memory, as well as her three-point orientation, were intact.
At no point did she have any thoughts of suicide or self-harm. She had good insight into her condition.
Physical examination was unremarkable. In particular, there was no neurological deficit.
Differential Diagnoses
•.
Chemotherapy-induced pseudobulbar affect
•.
Carboplatin-induced mania
•.
Dacrystic and Gelastic seizures
•.
Witzelsucht secondary to brain metastases
•.
Limbic encephalitis, panic disorder
Investigations
Her blood counts and electrolytes were essentially normal. Her liver function test and thyroid function test were also normal. Syphilis panel was unremarkable. Autoimmune antibodies were not done.
No abnormalities were detected in the magnetic resonance imaging (MRI) of her brain done after her presentation. Electroencephalogram (EEG) done was also unremarkable, with no epileptiform activity.
Impression
This is a 52-year-old woman, presenting with episodic emotional lability occurring only post-chemotherapy, with a background history of recently-diagnosed left breast carcinoma. Previously, she had undergone six cycles of chemotherapy with neoadjuvant steroid injection uneventfully, while on treatment for right breast carcinoma. The consultation–liaison psychiatry team elicited a notable pattern of occurrence for her symptoms, observed by the patient herself, as well as by her family members; specifically occurring on the second day post-chemotherapy, peaking over the 3rd and 4th day, and abating by the 6th day. Investigations done had been nonspecific. She has good insight into her symptoms.
Treatment
The patient was psycho-educated about her diagnosis and given reassurances regarding the self-limiting duration for her symptoms. She was also prescribed low-dose lorazepam, to be given as necessary when the symptoms occurred after every cycle of chemotherapy. She successfully completed the 6 cycles of chemotherapy planned. There were no side effects noted from treatment. Medical status was stable throughout and post-chemotherapy.
Discussion
As early as 1872, Charles Darwin observed that “certain brain diseases, such as hemiplegia, brain-wasting, and senile decay, have a special tendency to induce weeping.”
3 This condition was first described by Wilson
4 in a seminal study of the disorder in 1924, suggesting that bilateral lesions in the descending corticobulbar tracts lead to failure of voluntary control of emotion by the higher cortical centers.
This, in turn, leads to reduced function of the fasciorespiratory control center in the brainstem. The overall prevalence of PBA was estimated to be about 10% across six commonly-associated underlying neurological conditions (Alzheimer's disease, amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, stroke, and traumatic brain injury), and it appears to be under-recognized.
5 Among the varied sites of lesions noted, lenticulocapsular strokes were most strongly associated with the development of PBA.
Recent data suggest disruption of cortico–pontine–cerebellar circuits, reducing the threshold for motor expression of emotion.
6 Disruption of the microcircuitry of the cerebellum itself may likewise impair its ability to act as a gate-control for emotional expression. Current evidence also suggests that serotonergic and glutamatergic neurotransmission play key roles. Neurochemically, the circuits involved in PBA may be affected by drugs that modulate any of a variety of neurotransmitters.
6,7 Dysfunction of serotonergic, dopaminergic, and glutamatergic systems are most often suggested as the neurochemical foundations of this condition.
Until recently, the serotonergic hypothesis of PBA held sway, with much research done on the serotonergic pathways of the brain and the popular use of serotoninergic medications for treatment of this condition. However, in the late 1980s, the glutaminergic hypothesis became more prevalent in the consideration of PBA. As glutamate is the primary excitatory neurotransmitter in the central nervous system, including the circuits regulating affect, manipulating glutamate activity may help reduce PBA.
A strategy to afford such regulation of glutamate function might include the use of sigma−1 receptor agonists and/or uncompetitive
N-methyl-D-aspartate (NMDA) receptor antagonists. Sigma−1 receptors are concentrated in the brainstem and cerebellum, and limbic
10 and motor central nervous system, and their agonists have a rapid onset of action in modulating these neurotransmitters. By these mechanisms, sigma−1 receptor agonists such as dextromethorphan (DM) may improve regulation of affect.
8,9DM acts as a sigma−1 receptor agonist, and quinidine sulfate (Q) inhibits cytochrome P450 2D6, producing a 20-fold increase in circulating DM levels. The combination of DM 30 mg plus Q 30 mg has been shown to be effective in the treatment of PBA.
9Without a model for considering disorders of affect separately from disorders of mood, most patients with PBA, as was in this case, would almost certainly receive mood-disorder diagnoses (e.g., depressive, hypomanic, manic, or mixed mood episodes; major depressive disorder; and bipolar disorder diagnoses, including “ultra-rapid-cycling” bipolar disorder), when a diagnosis of PBA would have been more appropriate.
8Several scales may be of use in the identification and assessment of PBA, such as The Pathological Laughter and Crying Scale (PLACS), The Emotional Lability Questionnaire, The Affective Lability Scale, and The Center for Neurologic Study-Lability Scale (CNS-LS). The latter, which is self-administered, has been validated in patients with amyotrophic lateral sclerosis and multiple sclerosis. These scales assist in the screening for PBA and may also be valuable for the purpose of establishing a baseline severity level of PBA against which to assess the effects of treatment.
8As life expectancy lengthens, the broad variety of neurologic disorders underlying PBA will only become more common, creating a heightened need for PBA recognition and treatment.