Risk Factors for Psychosis Secondary to Temporal Lobe Epilepsy: A Systematic Review
Abstract
Methods
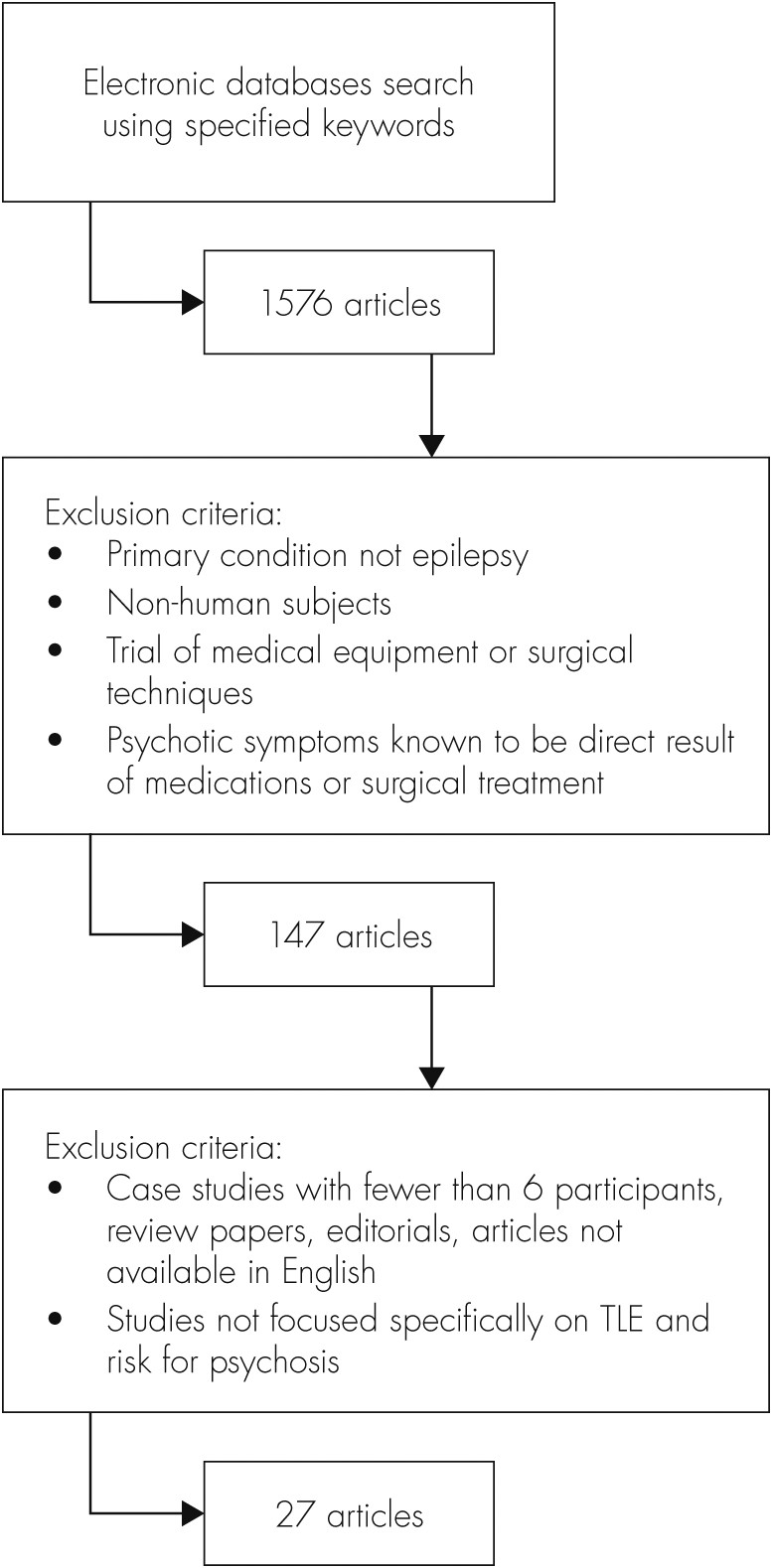
Results
| Quality Index Score | Author, Year, Country | Participants | Control Group/ Additional Experimental Group | Study Design | Method of Data Collection | Assessment of Psychosis | Factors Investigated | Statistical Analyses and Results |
|---|---|---|---|---|---|---|---|---|
| 12 | Adachi et al., 2002,25 Japan | 197 individuals with LRE (123 with TLE) and IIP | 456 individuals with LRE (288 with TLE) and NP | Case–control | Medical file review | Neuropsychiatric assessment using ICD–10 criteria | Age at epilepsy onset. | ANCOVA: |
| F=8.19; p=0.004* | ||||||||
| 12 | D’Alessio et al., 2009,17 Argentina | 63 individuals with TLE-P | 60 individuals with TLE-NP | Case–control | Medical file review | Psychiatric assessment using SCID-I and -II | t-tests: | |
| Gender; | p=0.039* | |||||||
| Employment; | p=0.001* | |||||||
| Unilateral HS; | NS | |||||||
| Bilateral HS; | p=0.001* | |||||||
| History of SE; | p=0.017* | |||||||
| Duration of epilepsy >20 yrs; | p=0.02* | |||||||
| Aura; | p=0.054 | |||||||
| Age at epilepsy onset | NS | |||||||
| 12 | Falip et al., 2009,10 Spain | 5 individuals with TLE-PIP | 50 individuals with TLE-NP | Case–control | Review of medical file | Psychiatric assessment | t-tests: | |
| Gender; | NS | |||||||
| History of febrile seizures; | NS | |||||||
| History of SE; | p=0.019* | |||||||
| Seizure frequency; | NS | |||||||
| Duration of epilepsy; | NS | |||||||
| Age at epilepsy onset; | NS | |||||||
| Family history of epilepsy; | NS | |||||||
| Dialeptic or automotor seizures evolving to secondary generalization; | p=0.025* | |||||||
| Lesion side; | χ2 tests: | |||||||
| Etiology; | NS | |||||||
| Nonlateral ictal EEG | NS | |||||||
| p=0.001* | ||||||||
| 12 | Kalinin et al., 2010,24 Russian Federation | 105 individuals with TLE | N/A | Case–control | Clinical and neuropsychological assessments and MRI | Psychiatric assessment using ICD–10 criteria | t-tests: | |
| Handedness; | Paranoia: p NS | |||||||
| Psychoticism: NS | ||||||||
| Laterality focus; | Paranoia: NS | |||||||
| Psychoticism: NS | ||||||||
| Alexithymia | Paranoia: p=0.002* Psychoticism: p=0.006* | |||||||
| 12 | Kanemoto et al., 2001,28 Japan | 132 individuals with epilepsy and IIP | 2,773 individuals with epilepsy and NP | Case–control | Medical file review | Psychiatric assessment using DSM-IV criteria | χ2 tests: | |
| Age at epilepsy onset (<10 years); | χ2=4.87; p <0.05* | |||||||
| Prolonged febrile seizures | χ2=13.73; p <0.01* | |||||||
| 11 | Briellmann et al., 2000,39 Australia | 6 individuals with TLE-PIP | 45 individuals with TLE-NP | Case–control | Collection of temporal lobe tissues | Psychiatric assessment using DSM-IV criteria | Volume loss of the anterior hippocampus; | Mann-Whitney U test: p=0.003*; |
| Mesial dysplasia | Chi square: p=0.006* | |||||||
| 11 | Cunha et al., 2003,37 Portugal | 18 medically-treated individuals with TLE; | N/A | Case–control | Clinical and neuropsychological assessment | Assessment of psychotic symptoms, using SCL–90 (self-administered) | Duration and severity of epilepsy | t-tests: |
| 19 surgically-treated individuals with TLE (examined pre- and postsurgery) | Paranoid Ideation: NS | |||||||
| Psychoticism: NS | ||||||||
| 11 | Kanemoto et al., 1996,27 Japan | 61 individuals with TLE and UHS (31 with PIP); | N/A | Case–control | Medical file review and MRI | Psychiatric assessment, using DSM-IV criteria | χ2 tests: | |
| 50 individuals with TLE with normal MRI (11 with PIP) | UHS; | χ2=9.7; p <0.01* | ||||||
| Age at epilepsy onset (<10 years); | χ2=5.53; p <0.05* | |||||||
| UHS, PIP, and atrophy of temporal neocortex | χ2=6.14; p <0.05* | |||||||
| 11 | Radhakrishnan et al., 2007,43 India | 129 individuals with surgically-treated TLE with CoA build-up; | N/A | Cross-sectional | Psychological and psychiatric assessment; EEG and MRI; collection of temporal lobe tissues | Psychiatric assessment, using ICD–10 criteria | t-tests: | |
| 244 individuals with surgically-treated TLE without CoA build-up | Frequency of IIP in CoA+ and CoA− groups; | p ≤0.001* | ||||||
| Frequency of IIP in Grades 1, 2, or 3 CoA | p=0.006* | |||||||
| 11 | Suckling et al., 2000,41 U.K. | 6 individuals with TLE-P | 26 individuals with TLE -NP | Case–control | Medical file review and collection of temporal lobe tissues | Neuropsychiatric assessment | Fisher’s exact test: | |
| Presence of focal lesions; | p=0.006* | |||||||
| Neuron loss in CA1; | p=0.015* | |||||||
| Neuron loss in CA4; | NS | |||||||
| Neuron loss in dentate gyrus; | NS | |||||||
| Dispersion of dentate granule cells | NS | |||||||
| 10 | De Araújo Filho et al., 2011,35 Brazil | 29 individuals with TLE | 6 individuals with JME | Cross-sectional | Medical file review | Psychiatric assessment, using DSM-IV criteria | Frequencies: 68.9% (20) | |
| 16 with IIP | 4 with IIP | Left-sided MTS; | ||||||
| 13 with PIP | 2 with PIP | Right-sided MTS; | 20.6% (6) | |||||
| Bilateral MTS | 10.3% (3) | |||||||
| 10 | De Oliveira et al., 2010,13 Brazil | 73 individuals with TLE | N/A | Cross-sectional | Clinical questionnaires | Mini-International Neuropsychiatric Interview (MINI) Plus, Version 5.0.0 | Bilateral MTS | Fisher’s exact test: NS |
| 10 | Flügel et al., 2006,26 U.K. | 20 individuals with TLE-IIP | 20 individuals with TLE-NP | Case–control | Neuropsychological assessments; MRI | Neuropsychiatric assessment, using DSM-IV criteria; PANSS | General linear model (multivariate): | |
| Age at onset of epilepsy; | F=10.3; p=0.003* | |||||||
| History of SE; | F=16.1, p=0.00* | |||||||
| Estimate of premorbid IQ; | F=1.4; NS | |||||||
| Current IQ; | F=3.16; NS | |||||||
| Vocabulary; | F=4.4; p=0.04* | |||||||
| Verbal Fluency (animals); | F=8.29; p=0.007* | |||||||
| Verbal Fluency (letters); | F=1.81; NS | |||||||
| Arithmetic; | F=5.09; p=0.03* | |||||||
| Digit Span; | F=3.02; NS | |||||||
| Spatial Span; | F=4.90; p=0.03* F=4.88; p=0.03* | |||||||
| Spatial Working Memory; | ||||||||
| Hippocampal volume | NS | |||||||
| 10 | Gutierrez-Galve et al., 2012,20 U.K. | 22 individuals with TLE-IIP | 23 individuals with TLE-NP; | Case–control | Medical file review and MRI | Neuropsychiatric assessment and PANSS | χ2 test: | |
| 21 healthy individuals | Gender; | χ2=0.58; NS | ||||||
| Handedness; | Fisher’s exact tests: | |||||||
| History of SE; | Fisher’s exact=0.345 | |||||||
| Total brain volume; | Fisher’s exact=0.004* | |||||||
| Age at epilepsy onset; | ANOVAs: | |||||||
| Duration of epilepsy; | F=7.92; p <0.001* | |||||||
| Estimates of Premorbid IQ; | t-tests: | |||||||
| Current IQ; | t = –2.62; p=0.012* | |||||||
| Working Memory Span; | t=1.61; NS | |||||||
| Working Memory Manipulation; | t = –1.06; NS | |||||||
| Frontal cortical thickness; | t = –2.44, p=0.019* | |||||||
| Cortical area; | t = –2.83; p=0.007* | |||||||
| Cortical volume | t=2.84; p=0.007* | |||||||
| ANOVAs: | ||||||||
| F=3.79; p=0.050* | ||||||||
| NS | ||||||||
| NS | ||||||||
| 10 | Kandratavicius et al., 2012,21 Brazil | 14 individuals with TLE-IIP | 16 individuals with TLE and no comorbidity; | Case–control | Collection of temporal lobe tissues | Psychiatric assessment, using DSM-IV criteria | ANOVAs: | |
| 16 individuals with TLE and major depression; | Gender; | NS | ||||||
| 10 normal autopsy samples | Presence of IPI; | NS | ||||||
| Age at epilepsy onset; | F=3.099; p=0.049* | |||||||
| Duration of epilepsy; | NS | |||||||
| Seizure frequency; | NS | |||||||
| Handedness; | NS | |||||||
| HS; | NS | |||||||
| Current IQ; | NS | |||||||
| Education (years); | NS | |||||||
| Seizure type; | Fisher’s exact tests: | |||||||
| Memory in Verbal Tasks; | p=0.02* | |||||||
| Neuronal density in entorhinal cortex; | p=0.003* | |||||||
| Fascia dentata inner molecular layer mossy fiber sprouting | ANOVAs: | |||||||
| F=3.175, p=0.047* | ||||||||
| TLE-IIP and TLE: | ||||||||
| F=4.931; Tukey’s post hoc: p=0.014* | ||||||||
| 10 | Rüsch et al., 2004,38 U.K. | 26 individuals with TLE-P | 24 individuals with TLE-NP; | Case–control | Medical file review and MRI | Neuropsychiatric assessment, using ICD–10 criteria | t-tests: | |
| 20 healthy individuals | Performance IQ; | t=0.203; NS | ||||||
| Verbal IQ; | t=2.307; p=0.02* | |||||||
| Current IQ; | t=1.902; p=0.06 | |||||||
| Cortical gray-matter volumes; | NS | |||||||
| Laterality of HS | NS | |||||||
| 10 | Sundram et al., 2010,30 Ireland | 10 individuals with TLE-P | 10 individuals with TLE-NP | Case–control | Medical file review and MRI | Neuropsychiatric assessment, which was objectively assessed using the OPCRIT | t-tests: | |
| Age at epilepsy onset; | NS | |||||||
| Duration of epilepsy; | NS | |||||||
| Total brain volume; | Mann-Whitney U tests: | |||||||
| Total gray-matter volume; | p=0.07 | |||||||
| Total white-matter volume; | p=0.08 | |||||||
| Gray-matter regional deficits; | NS | |||||||
| White-matter regional deficits | ANCOVAs: | |||||||
| Cluster significance threshold: p=0.002*; | ||||||||
| Cluster significance threshold: p=0.006* | ||||||||
| 10 | Tebartz van Elst et al., 2002,34 U.K. | 26 individuals with TLE-P | 24 individuals with TLE-NP; | Case–control | Medical file review and MRI | Neuropsychiatric assessment, using ICD–10 criteria | ANOVAs: | |
| 20 healthy individuals | Laterality of HS; | NS | ||||||
| EEG abnormalities; | NS | |||||||
| Total brain volumes; | F=11.750; p <0.001* | |||||||
| Hippocampal volumes; | NS | |||||||
| Right amygdala volumes; | F=8.211; p=0.001* | |||||||
| Left amygdala volumes. | F=9.079; p<0.001* | |||||||
| 10 | Umbricht et al., 1995,23 U.S.A. | 8 individuals with TLE-PIP; | 29 individuals with TLE-NP | Case–control | Medical file review | Psychiatric assessment, using DSM-III-R criteria | ANOVAs: | |
| 7 individuals with TLE-IIP | Duration of epilepsy; | NS | ||||||
| Bilateral focus; | Fisher’s exact test: p <0.005* | |||||||
| Seizure clusters; | χ2 tests: χ2=3.75; p=0.05* | |||||||
| Febrile convulsions; | χ2=4.36; p <0.05* | |||||||
| Handedness; | p>0.05 | |||||||
| Age at epilepsy onset; | t-tests: | |||||||
| Time between first seizure and onset of epilepsy; | t=2.67; p <0.05* | |||||||
| Full-scale IQ; | t=2.81; p <0.01* | |||||||
| Verbal IQ. | Mann-Whitney U tests: | |||||||
| z=1.98; p <0.05* | ||||||||
| z=2.11; p <0.05* | ||||||||
| 9 | De Araújo Filho et al., 2008,33 Brazil | 170 individuals with TLE | 100 individuals with JME | Cross-sectional | Medical file review and EEG monitoring | Psychiatric assessment, using SCID-I | Left-sided MTS | χ2 test: p <0.05* |
| 9 | Flügel et al., 2006,18 U.K. | 20 individuals with TLE-IIP | 20 individuals with TLE-NP; | Case–control | Clinical assessments; MRI | Psychiatric assessment, using DSM-IV criteria; PANSS | Mann-Whitney U tests: | |
| 23 healthy individuals | Gender; | Z = –0.64; NS | ||||||
| Estimated Premorbid IQ; | Z = –0.92; NS | |||||||
| Age at epilepsy onset; | Z = –2.91; p=0.004* | |||||||
| Min. and max. seizure frequency; | Z = –0.56; NS | |||||||
| MTR in the left middle and superior temporal gyri | p ≤0.005* | |||||||
| 9 | Flügel et al., 2006,48 U.K. | 20 individuals with TLE-IIP | 20 individuals with TLE-NP | Case–control | Neuropsychological assessments and MRI | Neuropsychiatric assessment; PANSS | General linear model (multivariate): | |
| FA values in frontal left; | F=5.54; p=0.024* | |||||||
| FA values in frontal right; | F=12.18; p=0.001* | |||||||
| FA values in temporal left; | F=5.89; p=0.02* | |||||||
| FA values in temporal right; | F=6.295; p=0.017* | |||||||
| MD values in frontal left; | F=5.203; p=0.029* | |||||||
| MD values in frontal right | F=5.88; p=0.02* | |||||||
| 9 | Fukao et al., 2009,36 Japan | 16 individuals with TLE-P | 41 individuals with TLE-NP | Case–control | Medical file review and magnetoencephalographic measurements | Psychiatric assessment, using DSM-IV criteria | Correlation: | |
| Laterality of focus; | NS | |||||||
| IH-type spike-dipoles; | NS | |||||||
| Left SV-type spike-dipoles; | p=0.002* | |||||||
| Plural types of spike-dipoles; | p=0.001* | |||||||
| Bilateral magnetic spikes; | p=0.046* | |||||||
| MTS | NS | |||||||
| 9 | Gattaz et al., 2011,8 Brazil | 7 individuals with TLE-IIP | 9 individuals with TLE-NP | Case–control | Collection of temporal lobe tissues | Psychiatric assessment, using DSM-IV criteria | Mann-Whitney U tests: | |
| iPLA2 activity; | p=0.016* | |||||||
| cPLA2 activity; | NS | |||||||
| sPLA2 activity; | NS | |||||||
| tPLA2 activity; | NS | |||||||
| Duration of epilepsy; | NS | |||||||
| Frequency of seizures | NS | |||||||
| 9 | Hermann et al., 2000,29 U.S.A. | 54 individuals with TLE | 38 healthy individuals | Case–control | Neuropsychological questionnaires | Assessment of psychotic symptoms, using SCL–90-R | Partial correlation: | |
| Duration of epilepsy; | Paranoid Ideation r=0.46; p=0.001* | |||||||
| Frequency of seizures | Psychoticism: r=0.40; p=0.004* | |||||||
| NS | ||||||||
| 8 | Guarnieri et al., 2005,19 Brazil | 21 individuals with TLE | 23 individuals with TLE-NP | Case–control | SPECT scans | Psychiatric assessment, using DSM-IV criteria | χ2 tests: | |
| 11 with IIP | Gender; | χ2=0.349, NS | ||||||
| 10 with PIP | Marital status; | χ2=1.85; NS | ||||||
| Handedness; | χ2=0.934; NS | |||||||
| Presence of IPI; | χ2=0.02; NS | |||||||
| Resonance laterality; | χ2=0.380; NS | |||||||
| EEG ictal laterality; | χ2=0.509; NS | |||||||
| Education (years); | t-tests: | |||||||
| Age at epilepsy onset; | t=0.102; NS | |||||||
| Duration of epilepsy; | t=0.046; NS | |||||||
| Paid employment; | t=0.480; NS | |||||||
| Seizure frequency; | Fisher’s exact test: | |||||||
| rCBF | F=2.53; NS | |||||||
| Mann-Whitney U test: | ||||||||
| U=214.0; NS | ||||||||
| ANOVA; NS | ||||||||
| 6 | Maier et al., 2000,42 U.K. | 12 individuals with TLE-P | 12 individuals with TLE-NP; | Case–control | Medical file review and MRI | Psychiatric assessment | t-tests (TLE-P and HCs): | |
| 26 individuals with schizophrenia and no epilepsy; | Left NAA; | p <0.001* | ||||||
| 38 healthy individuals | Right NAA; | p <0.001* | ||||||
| Left Cho; | NS | |||||||
| Right Cho; | NS | |||||||
| Left Cr+PCr; | NS | |||||||
| Right Cr+PCr; | NS | |||||||
| Left regional H/A volume; | p=0.0001* | |||||||
| Right regional H/A volume | p=0.004* |
Quality of Studies
Gender
Handedness
Age at Epilepsy Onset
Seizure History
Seizure Laterality
Seizure Frequency
The Limbic System
Temporal and Extratemporal Features
Cognitive Functioning
Personality Factors
Employment
Discussion
| Risk Factor | PIP | IIP | Psychosis Not Ictally-Defined |
|---|---|---|---|
| Early onset of epilepsy (before age 10) | *** | *** | |
| History of prolonged febrile convulsions | *** | ||
| History of status epilepticus | *** | *** | *** |
| Dialeptic and/or automotor seizures | *** | ||
| Left-sided mesial temporal sclerosis | *** | ||
| Bitemporal seizures or seizures spreading quickly to the contralateral temporal lobe | *** | ||
| Secondarily generalized seizures | *** | ||
| Bitemporal seizure foci | *** | ||
| Seizure clusters | *** | ||
| Bilateral hippocampal white-matter loss | *** | ||
| Left hippocampal gray-matter reduction | *** | ||
| Neuron loss in CA1 region of hippocampus | *** | *** | †*** |
| Bilateral hippocampal sclerosis | *** | ||
| Unilateral hippocampal sclerosis | *** | †*** | |
| Reduced synaptic activity in left hippocampus | ** | ||
| Decreased synaptic reorganization in dentate gyrus | *** | ||
| Higher iPLA2 activity in hippocampus | ** | ||
| Regional volume-reduction in hippocampus/amygdala | ** †*** | ||
| Dysplastic malformations or atrophy of temporal neocortex | *** | ||
| Neural abnormalities in left middle and superior temporal gyri | ** | ||
| Axonal loss in temporal & frontal lobes, especially right frontal lobe | ** | ||
| Extratemporal regional gray- and white-matter deficits | *** | ||
| Lower current IQ | *** †*** | *** †*** | |
| Alexithymia | *** |
Limitations of the Current Review
Recommendations
Definition, Diagnosis, and Classification of Psychosis
Study Populations
Study Design
Conclusions
Acknowledgments
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

