Violence by juvenile offenders is a costly problem that has important social relevance for the political, criminal justice, and health care systems.
1,2 Previous work has suggested that more than half a million juveniles worldwide are detained in custody,
3 furthermore, within the United States, one-third of the offenders arrested for a violent offense are under 18 years,
4 suggesting that juvenile offenders are responsible for a disproportionate amount of violent crime. In addition, between $82 million US and $103 million US is spent each year in just one US city on victim costs arising from the violence perpetrated by juvenile offenders.
5 The early identification and management of those juveniles at greatest risk of violence may, therefore, have benefits not only for the offender but also for society through reductions in victim and associated costs.
6Given its ability to link concurrent brain changes with behavioral outcomes,
7 neuroimaging research offers one method of identifying individuals at risk of violence. As maturation of the brain occurs in a heterochronous manner,
8 imbalance in the maturation of subcortical structures relative to the development of the prefrontal cortex has been proposed as one mechanism that underlies the development of risk-taking behaviors,
9 including violence,
10 in juveniles. In line with this proposition, certain subcortical structures, including the caudate and amygdala, are of significantly increased volume in male children (between 7 and 11 years of age) compared with adult males (between 17 and 33 years of age).
11 The prefrontal region, in contrast, while not of significantly reduced volume in adolescents, does exhibit significant changes in neuronal density in adolescents (between 12 and 16 years of age) compared with adults (between 23 and 30 years of age).
12These structural differences may also result in differential functioning between limbic and prefrontal regions. Functional MRI (fMRI) research, for example, has found that during a reward processing task, activation in the accumbens was exaggerated while orbitofrontal cortical activity was significantly decreased in adolescents (between 13 and 17 years of age) compared with adults (between 23 and 29 years of age).
13 While subcortical regions of the brain, including the caudate, amygdala, and accumbens underlie the production of emotional responses, prefrontal structures are thought to exert cognitive control over the expression of these emotions.
14 Exaggerated activity in these subcortical structures, without adequate activity in the prefrontal cortex may underscore the expression of impulsive, emotionally driven behaviors, including violent behaviors.
15 Reviews of previous volumetric and functional neuroimaging work, for example, have concluded that violent adults can be distinguished from nonviolent adults by reduced volumes and activity in the prefrontal cortex
16 as well as increased subcortical activity.
17Much neuroimaging research into the development of the frontal-subcortical circuits, however, has focused on age-related changes in subcortical and prefrontal structures or activity and has, therefore, typically compared volumetric or functional differences in these brain regions between healthy adolescents and adults.
7 In addition, previous work has investigated aberrations in functional connectivity between brain regions in the frontal-subcortical circuits. Therefore, whether violent juveniles may be characterized by delayed maturation within brain regions of the frontal-subcortical circuit relative to healthy peers is unknown at present. Local functional connectivity techniques may provide greater detail as to the patterns of activity within specific regions.
18Resting-state functional connectivity MRI (Rs-fMRI) techniques can be used to study the construction of intrinsic, interconnected neural circuits, and their relation to other cognitive or behavioral states.
19 The regional homogeneity (ReHo) method is most frequently used to investigate Rs-fMRI local connectivity.
20 This method assumes that for a given voxel, resting-state activity is correlated with that of functionally related neighboring voxels.
21 ReHo is, therefore, thought to reflect coordination in regional neural activity.
22 In addition, the ReHo method measures local functional connectivity within brain regions
18 on a voxel-wise basis and, as such, enables investigation of close-range functional connectivity within brain regions.
23 The present study, therefore, used the ReHo method to investigate whether violent juvenile offenders show any differences local functional connectivity, which may underscore the development of violence. Based on structural and functional neuroimaging work in adults,
16,17 we expected violent juvenile offenders would show greater ReHo levels in subcortical regions and lower ReHo values in prefrontal regions compared with healthy age-matched controls.
Methods
As previous work has indicated that patterns of brain maturation differ between male and female juveniles,
11 we only included males in the present study. A total of 30 violent male juvenile offenders and 29 controls were recruited to participate. All participants were aged between 15 and 17 years, were right-handed, and had no history of neurological impairment, including: paralysis, loss of sensation, muscular weakness, epilepsy, seizures, chronic pain, confusion, or altered levels of consciousness.
Violent offenders were recruited from the Hunan province Youth Detention Center (YDC) in the People’s Republic of China. They were recruited based on the severity of their index offense as recorded from official police records. Specifically, all violent juvenile offenders had been convicted of homicide or assault.
The Chinese version of the Schedule for Affective Disorder and Schizophrenia for School-Age Children Present and Lifetime (K-SADS-PL)
24,25 was used to assess current and lifetime psychiatric problems according to DSM-IV criteria. The K-SADS-PL interview procedure is outlined in detail elsewhere.
26 On the basis of this assessment, any participant diagnosed with any current or lifetime psychiatric disorder was excluded. In addition, all participants had to be free from substance misuse within the last 3 months according to urine analysis, self-report, and family informant report.
Oral and written information about the aims, content, and duration of the study was given to all participants. Participants were also informed that their information was confidential and that refusal to participate would not affect their judicial status or stay in the YDC. Written informed consent was required from all the participants and from their legal guardians. All discussions about study participation were conducted in a private area of the YDC. No compensation was given for participation.
The procedures of this study were approved by the Biomedical Ethics Board of the second Xiangya Hospital, Central South University, People’s Republic of China.
MR Imaging
MR images were acquired on a Siemens Allegra 3.0 T MR scanner at the Magnetic Resonance Center of Hunan Provincial People’s Hospital, People’s Republic of China. A standard birdcage head coil was used, along with restraining foam pads to minimize head motion and to diminish the sounds of the scanner. Rs-fMRI scans were performed by an echo planar imaging (EPI) sequence with scan parameters of in-plane resolution=64×64, repetition time=3000 ms, echo time=30 ms, flip angle=90, field of view=240×240 mm2, slice thickness of 3 mm and no gap. Each brain volume comprised 32 axial slices, and each functional run contained 100 volumes. During the Rs-fMRI scan, participants were instructed to keep their eyes closed, relax, and move as little as possible.
Image Analysis
The first 10 volumes of each functional time series were discarded from analysis because of possible instability of the initial signal and movement caused by the participants’ adaptation to the scanning environment. The remaining 90 volumes were used in subsequent analyses. Image preprocessing, including slice timing, head motion correction, and spatial normalization, were conducted using statistical parametric mapping software SPM8 (
http://www.fil.ion.ucl.ac.uk/spm) based on Matlab 7.8.
Head motion parameters were computed by estimating the translation in each direction and the angular rotation on each axis for each volume. Any subject who had a maximum displacement in any of the cardinal directions (x, y, z) that was larger than 2 mm, or 2° of angular motion, during the whole fMRI scan were excluded.
After slice acquisition correction and head motion correction, fMRI data were normalized to the standard SPM8 echo-planar imaging template and interpolated to 3×3 ×3 mm
3 voxels. Then, using the in-house RESTV 1.6 analysis toolkit (
http://resting-fmri.sourceforge.net),
27 images were band-pass filtered (0.01 Hz<f<0.08 Hz) to reduce low-frequency drift and physiological high-frequency respiratory and cardiac noise.
28 To reduce the influence of rising temperature from the MRI equipment, linear trends were also removed.
ReHo analysis was performed with in-house software REST V1.6.
27 Individual ReHo maps were generated by calculating Kendall’s coefficient of concordance (KCC),
20 which compares the temporal firing sequence of a given voxel to those of its 26 nearest neighbors in a voxel-wise analysis. The individual ReHo maps were smoothed using a Gaussian kernel of 8 mm full width at half-maximum. Maps were then subjected to group statistical analysis using SPM8.
Statistical Analysis
Comparison between the violent juvenile offenders and controls was performed using chi-square tests for categorical variables and t tests for continuous variables. Differences were considered significant when p <0.05. All analyses were conducted using SPSS, version 17.0.
Two-sample
t test was performed on the individual ReHo maps in a voxel-by-voxel manner for all between-group comparisons. The resulting statistical map was corrected following an approach used previously.
29 In brief, the threshold used for each individual voxel was as follows: p value of <0.005, cluster sizes >324 mm
3 (12 voxels).
Brain regions displaying significant differences between the two groups were identified as regions of interest (ROIs). Mean ReHo values of each ROI for each subject were then extracted using an approach described in greater detail elsewhere.
30Following an approach used previously,
29 area under the receiver operating characteristic curve (AUC) analyses were used to investigate the sensitivity and specificity of detected abnormalities in ReHo values in order to compare their effectiveness in identifying the violent juvenile offenders. Adopting guidelines proposed by Swets (1988),
31 AUC values were interpreted as excellent if ≥0.90; good if 0.90 >AUC ≥0.80; fair if 0.80 >AUC ≥0.70; poor if 0.70 >AUC ≥0.60; and as having no effect if AUC <0.60. AUC analyses were also conducted using SPSS, version 17.0 (SPSS Inc).
In addition, following previous methods,
32 Fisher discriminant analysis was used to predict whether a participant was a violent offender or a control according to the individual’s ReHo values for those ROIs which demonstrated significant (p <0.005) differences in ReHo values between groups. The discriminant analysis was then cross-validated using the leave-one-out method.
32Results
Information from two participants had to be excluded from the analysis owing to excessive head movement: one each from the violent juvenile offender and control groups. Among the remaining 29 juvenile offenders, four (13.8%) were convicted of homicide and 25 (86.2%) were convicted of assault occasioning actual bodily harm.
The duration of education of the offenders was significantly lower than that of the controls (7.7±2.2 years versus 10.0±0.0 years; t=5.73, df=55, p <0.01). There were no significant age differences between the violent juvenile offenders and the controls, however, (=16.0±0.7 years versus =16.0±0.4 years; t= 0.08, df=55, p=0.99).
ReHo: Comparisons Between Violent Offenders and Controls
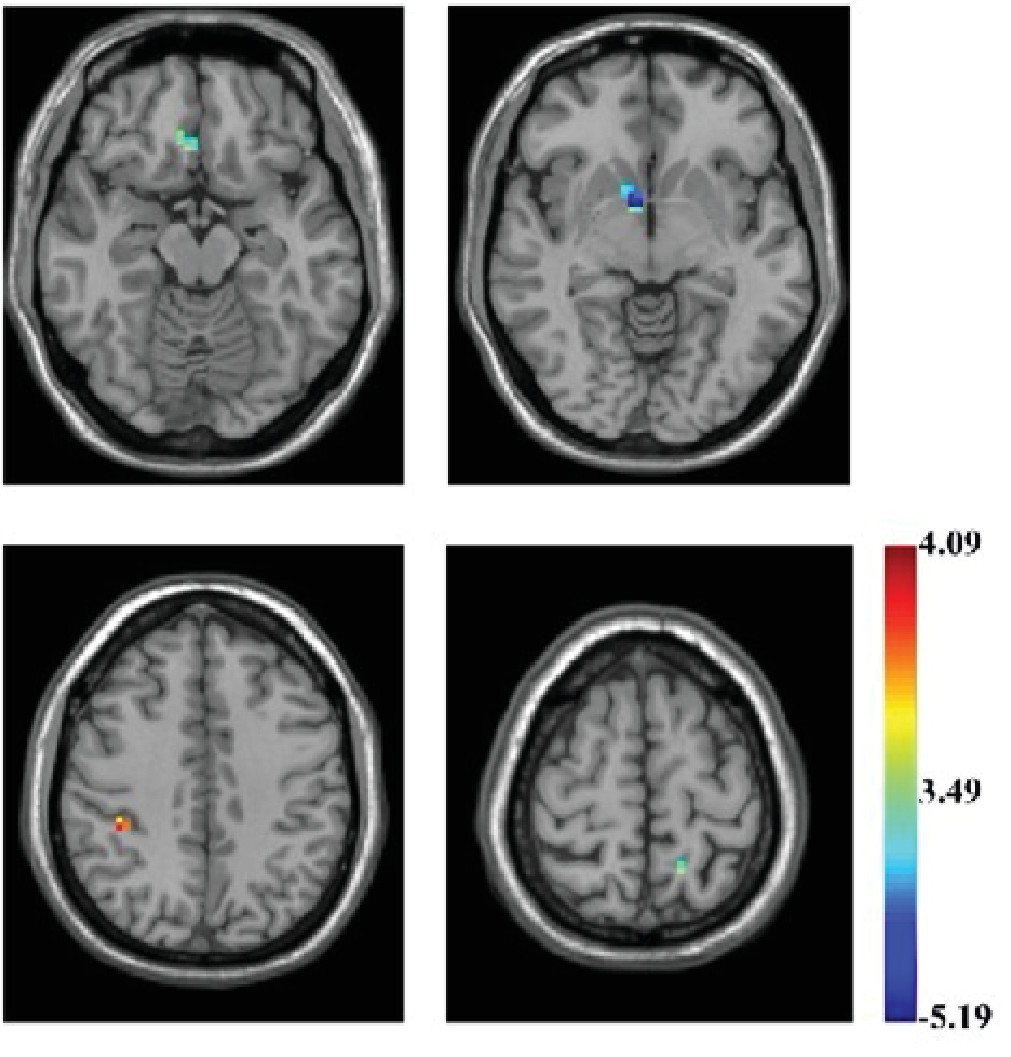
Violent juvenile offenders showed significantly lower ReHo values in the right caudate (Montreal Neurological Institute (MNI) coordinate [9,6,–9],
t=–5.186, corrected p <0.01), right medial prefrontal cortex (r-mPFC) (MNI coordinate [6,30,–15],
t=–3.994, corrected p <0.01), left precuneus (MNI coordinate [–15,–51,66],
t=–4.463, corrected p <0.01), and significantly higher values in the right supramarginal gyrus (MNI coordinate [39,–36,45],
t=4.08, corrected p <0.01) (
Table 1 and
Figure 1).
ROC Analysis and Discriminant Analysis: Abnormalities in ReHo Values Between Violent Offenders and Controls
Abnormalities in ReHo values for the caudate, precuneus, mPFC, and supramarginal gyrus had high sensitivity and specificity in distinguishing between the violent offenders and controls (
Table 2). For each of these four areas, the AUC
33 was higher than 70% suggesting that dysfunction in these areas represents a fair biomarker of violence in juveniles.
The four areas that showed ReHo abnormalities between the two groups were then subjected to discriminant analysis. The total percentage of correct classification was 89.5%, suggesting that 89.5% of all participants were classified into the correct groups: 85.7% were correctly classified as typically developing controls, whereas 93.1% were correctly classified as violent juvenile offenders. The validity of ReHo abnormalities in differentiating between the two groups was supported by Wilk’s lambda=0.46 (df=4, p<0.001).
Discussion
To our knowledge, this study represents the first attempt to use the ReHo method as an addition to Rs-fMRI data to identify neural correlates of violence in juveniles. We found that, compared with typically developing controls, male violent juvenile offenders had lower ReHo values in the right mPFC, right caudate, and left precuneus, coupled with higher ReHo values in the right supramarginal gyrus. These findings provide primary evidence of abnormal spontaneous activity specific to the prefrontal-striatal circuit in male violent juvenile offenders.
Throughout adolescence and into early adulthood, the brain undergoes a period of maturation such that prefrontal regions become increasingly active while subcortical regions become less active.
34 fMRI work suggests that subcortical structures, and most notably the amygdala, are implicated in processing of emotional material, whereas the prefrontal cortex is implicated in the cognitive interpretation of emotion.
14 In this way, prefrontal structures are thought to directly regulate activation in the subcortical system.
15 Maturational changes in the functional circuitry of the subcortical and prefrontal systems, therefore, enable the development of cognitive control, helping adolescents to suppress the inappropriate expression of emotions to achieve a more mature cognitive style.
34 Delayed, or even incomplete, maturation of this circuit, in contrast, may underlie the development of violence.
Adults who have sustained damage to the frontal lobes, for example, are unable to suppress the behavioral expression of emotion and may instead respond with exaggerated or even inappropriate emotional reactions.
35 Frontal lobe damage has also been implicated with the development of violence.
35 Reduced volume or function in the prefrontal cortex, even in the absence of damage, has also been associated with increased violence, particularly impulsive violence.
36 In addition, volumetric and functional neuroimaging research has found that violent adults are distinguished by reduced volumes and activity in the prefrontal cortex,
16 alongside increased subcortical activity.
17Much of the neuroimaging research into the development of the brain compares differences between healthy adolescents and adults.
7 In one exception, task-dependent fMRI was used to investigate functioning in the frontal-striatal circuit in male adolescents (between 12 and 15 years of age) diagnosed with externalizing disorders compared with healthy age-matched controls. Male adolescents diagnosed with externalizing disorders showed poorer bottom-up connectivity from the caudate to the middle frontal cortex, coupled with an absence of top-down connectivity running from the middle frontal cortex to the caudate.
37Our study, however, represents the first attempt to use Rs-fMRI ReHo analysis to compare male violent juvenile offenders with their typically developing peers. Both the low local connectivity of the right mPFC and caudate indicates that dysfunction may be localized to the right medial prefrontal-striatal circuit and that this circuit may demonstrate not only abnormal task-related activity,
37 but also abnormal spontaneous activity.
Given the significant association between attention deficit hyperactivity disorder (ADHD) and violence in juveniles and young adults,
38 and that dysfunction in the frontal-striatal circuit has also been observed in patients with ADHD compared with healthy controls,
39 it is of interest to note that the four ROIs identified in this study appear distinct from those implicated in ADHD.
40 We also evaluated the sensitivity and specificity of these ReHo abnormalities to investigate whether dysfunction in this circuit was sensitive and specific to male violent juvenile offenders compared with their healthy peers. AUCs for dysfunction in each of the regions identified by ReHo analysis were specific to the male violent juvenile offenders. Discriminant analysis, furthermore, suggested that dysfunction in ReHo values in this circuit could predict which participant was a member of the violent offender group. Taken together then, results of this study suggest that dysfunction in the prefrontal-striatal circuit during the resting state may be indicative of brain immaturity, which is specific to male violent juvenile offenders.
Results of these analyses extend previous work which found that neuropsychological methods are significantly more accurate at distinguishing between male violent and nonviolent adult offenders than personality measures.
41 ReHo measurements in these regions, therefore, appear to be promising neuroimaging biomarkers for characterizing violent male juveniles. Although previous work indicates that ReHo value abnormalities can be used to predict brain immaturity in individual participants,
42 further work will be necessary to validate these findings, however, before ReHo can be used to identify individual juveniles at risk of violence.
Limitations and Implications
Our study is limited in some respects. First, the study was conducted solely in boys, so findings cannot be generalized to violent girls or to adults. Further work is required to establish whether female violent juvenile offenders are characterized by the same pattern of dysfunction.
Second, other etiological factors may have confounded the pattern of brain dysfunction observed in this study. For example, as most of the violent juvenile offenders who participated in this study had lower education levels compared with the typically developing controls, a lack of education may be responsible for the delays in brain maturity observed in this study. Further work could investigate whether educational exposure influences brain development such that nonviolent juveniles with a lower educational level exhibit the same pattern of dysfunction as the violent offenders in this study.
Third, previous work has also shown that juvenile offenders are significantly more likely than their noncriminally involved peers to sustain a traumatic brain injury.
43 Longitudinal work is, therefore, necessary to determine whether the patterns of brain dysfunction identified in this study are a cause or a consequence of violence.
In addition, as the precuneus is one of the most highly active areas of the brain during the resting state,
44 it is unclear whether this structure should be considered a biomarker of violence at this stage.
Last, because of power considerations, we were unable to divide our sample into training and validation sets for the purposes of discriminant analysis. Further work using a fresh sample is, therefore, necessary to investigate whether the four ROIs identified in this study distinguish between male controls and violent juvenile offenders with the same degree of correct classification as found in this study.
Conclusions
This study represents a first step in determining local functional connectivity in violent juvenile offenders compared with controls. The ReHo method used to analyze voxel-based whole-brain resting-state activity in male violent juvenile offenders revealed that, compared with typically developing controls, male violent juvenile offenders were characterized by significantly lower ReHo values in the right caudate, right mPFC, and left precuneus, and significantly higher values in the right supramarginal gyrus. AUC analyses, alongside discriminant analyses, revealed that these abnormalities were associated with high sensitivity and specificity suggesting that right medial prefrontal-caudate circuit dysfunction maybe an important biomarker of immaturity in male violent juveniles and that the ReHo is a promising method of detecting these abnormalities during the resting-state.