Knowledge about the oxytocin (OT) system in the brain has increased greatly over the past decade.
15 Although this neuropeptide is best known for its peripheral effects, direct modulation of central nervous system (CNS) areas has also been implicated in OT’s actions, which include a major role in a wide range of affiliative behaviors.
16–24 Often referred to as the “social bonding” hormone, speculations are being made as to its applications and potential uses in enhancing human relationships. Alterations in the OT system have been implicated in several neuropsychiatric disorders.
25 Multiple types of psychopathology manifest in deficits in social functioning, including inability to maintain interpersonal relationships and engage in socially appropriate behavior. The OT system may influence the efficacy of psychotherapy, as research has repeatedly shown that the therapeutic relationship is one of the largest predictors of therapeutic change.
26 OT may also have value as a therapeutic intervention.
CNS Anatomy
OT is a nine amino acid peptide, synthesized primarily in the supraoptic and paraventricular nuclei (SON and PVN) of the hypothalamus.
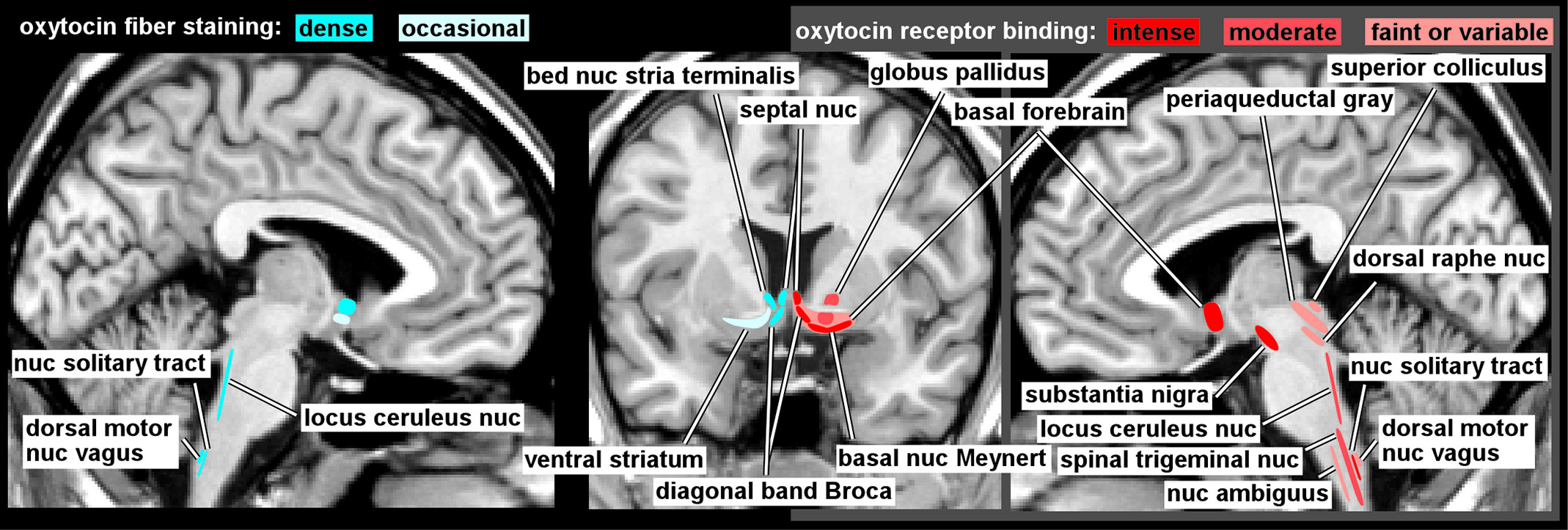
1,18 Neurons in both the SON and PVN project to the posterior pituitary gland, where OT is released into the bloodstream in response to specific physiological events (e.g., sexual stimulation, nursing, stress) and exerts multiple peripheral effects. OT neurons also send projections to (as indicated by staining of fibers) and OT receptors are present in (as indicated by specific binding) other regions of the CNS in species-specific distributions.
1,2 The few studies of OT receptor binding in human brain (
Figure 1) indicate considerable differences from rodent brain.
1,3,4 Of particular relevance is the absence in human brain of the extensive binding in limbic-related areas (e.g., amygdala, hippocampus, subiculum, entorhinal cortex, bed nucleus of the stria terminalis) that is prominent in the rodent. In contrast, high levels of binding were present in parts of the human basal forebrain (e.g., basal nucleus of Meynert, vertical limb of the diagonal band of Broca) and in substantia nigra that are not found in rodent. Both species have binding in the lateral septal nucleus, ventral striatum, and in multiple areas of brainstem and spinal cord. Animal studies indicate that OT receptor distribution is both species- and strain-specific and correlates with behavioral differences.
18,23,27 The few available studies of OT fiber staining in human brain (
Figure 1) suggest a generally similar pattern to the receptor mapping, with fibers present in the basal forebrain (e.g., septal nucleus, diagonal band of Broca, bed nucleus of the stria terminalis) and brainstem, but not in amygdala or hippocampus.
5–7 These differences should be borne in mind when applying results from other species to interpretation of human studies or application to human behaviors.
An intriguing aspect of the OT system in the CNS is the presence of areas with substantial OT receptor binding but low levels of fibers staining for OT, such as the substantia nigra pars compacta.
1,5 It has been hypothesized that such mismatches indicate the presence of volume transmission as an important mode of OT action.
28,29 OT, like other neuropeptides, is stored in large dense-core vesicles which are found in and can release OT from multiple locations (e.g., nerve endings, dendrites, soma, axonal varicosities).
23 Dendritic release by hypothalamic neurons is believed to be a major source of OT in the cerebral spinal fluid (CSF).
23,28 CSF and plasma OT levels are different and appear to be mostly independently regulated. The half-life of OT is much longer in CSF (∼28 minutes) than in the blood (1–2 minutes), suggesting a very different time-scale for CNS effects.
28 Unlike circulating OT, which does not easily cross the blood–brain barrier, OT in CSF has been suggested to reach multiple brain areas and to thereby alter physiology and behavior.
28Peripheral Oxytocin
Numerous studies in humans have reported correlations between peripheral levels of OT (i.e., in blood, saliva, or urine) and socially-relevant behaviors.
16,19,22,24,25 No correlation between urinary and plasma OT has been reported, suggesting that urine sampling may not be reliable marker of circulating OT.
30,31 In most studies, OT is measured by assay of OT concentration in the blood or saliva, and the two are shown to correlate to a certain extent.
30–32 However, OT has also been shown to be synthesized and released at peripheral sites, including the heart, thymus gland, gastrointestinal tract, uterus, placenta, amnion, corpus luteum, and testes.
1,20In humans, the most notable peripheral OT release is related to childbirth, breast-feeding, and copulation.
16,22,33 Peripheral OT has been correlated with many aspects of parent–infant bonding.
22 Plasma OT levels of pregnant women in the first trimester predict the amount of postpartum maternal–infant bonding behavior, and the increase in OT from the first to third trimester predicts third-trimester strength of maternal bonding (Maternal–Fetal Attachment Scale), suggesting that OT in gestation functions to prime the expression of maternal behavior.
22 Emerging research suggests that disruptions in maternal–infant bonding are marked by dysregulation of the OT system. Mothers at higher risk for postpartum depression, based on self-reported symptoms pre- and postpartum, had lower plasma OT during pregnancy.
34 Measures of adult attachment and temperament correlated with maternal differences in the increase in OT induced by interacting with their infants, and with brain activation patterns (measured with functional magnetic resonance imaging, fMRI) while viewing pictures of their own or other infants.
35,36 When viewing their own infant’s happy face, secure mothers showed greater activation in ventral striatum, orbitofrontal cortex, and medial prefrontal cortex, whereas insecure mothers showed greater activation in the dorsolateral prefrontal cortex.
35 The authors suggested that these differences indicate inhibition of negative affect by the insecure mothers, which may correspond with a disruption of the OT system that presumably supports parent–child empathy. Other studies have shown that both basal levels as well as reactivity OT measured in plasma and saliva of parents is associated with gender-specific parental behaviors such as affectionate touch in mothers and stimulatory touch in fathers, suggesting that touch-based interventions might be efficacious when the OT system is dysfunctional (e.g., maternal postpartum depression).
22 Similarly, a recent study found that increases in key paternal parenting behaviors (e.g., touch, social gaze, social reciprocity) were paralleled by increases in infants’ engagement behaviors (e.g., social gaze, exploration, social reciprocity) and peripheral OT levels, suggesting possible translational implications for an indirect neuropeptide treatment of infants at risk for social dysfunctions, such as siblings of children with autism-spectrum disorders.
37 Parents who are unable to attune to their child may contribute to dysfunctional development of the child’s OT system. Lower levels of OT in response to interactions with caregivers were found among adopted children with a history of disrupted caregiving, although basal levels were normal.
38 A recent study of personality traits, that included neuroimaging, found a positive correlation with extraversion (higher OT, higher extraversion) and a negative correlation with volume of the right amygdala/hippocampus complex (higher OT, smaller lateral amygdala and anterior hippocampus).
39 The authors noted that the lateralization is consistent with preferential involvement of the right hemisphere in modulation of social and emotional behavior, and they suggest that interaction of social experience with genetic factors may be involved.
Some studies have found that peripheral OT levels are significantly increased in the early stages of romantic attachment and that OT levels correlated with positive behaviors (e.g., positive communication, emotional matching, affectionate touch, interpersonal focus, and emotional support between partners).
40–42 Interestingly, OT levels among new lovers were significantly higher even than those observed in new parents and were modestly predictive of relationship stability at 6-month follow-up.
42 However, plasma OT also correlated with new lovers' preoccupations and worries regarding the partner and the relationship, providing evidence for a possible role of OT in anxious attachment. Similarly, several studies have reported positive correlations between OT and relational distress (e.g., anxiety, anxious attachment, distressful romantic relationships).
43–45 In a recent large, healthy-cohort study (N=473), trait anxiety and attachment anxiety were negatively correlated with plasma OT levels in men, consistent with its proposed anxiolytic effects.
46 In contrast, attachment anxiety was positively correlated with OT in women, and no association was found between OT and trait anxiety. Also, women with extreme values of OT were much more likely to self-report being highly anxious on a daily basis, suggesting that severe anxiety symptomology in women may be linked with an over-activation of the OT system.
46Altered levels of peripheral OT have also been correlated with presence of several neuropsychiatric disorders. Lower levels have been reported in some studies of depression, autism-spectrum disorders (ASD), and schizophrenia, although findings do vary.
19,24,25,47,48 In contrast, a positive correlation was found between OT and symptom severity in patients with social anxiety disorder (SAD).
25 It has been suggested that peripheral OT level may indicate an individual’s sensitivity to socially-relevant information.
49 Although studies finding significant correlations between peripheral OT and many aspects of human affiliative behaviors are intriguing, disentangling peripheral and central sites of action is complex.
16,20 The finding that intranasally-administered neuropeptides (melanocortin, vasopressin, insulin) reach the CSF led to a surge of studies demonstrating the impact of exogenous OT administration on a host of social behaviors and cognitions in humans.
50Intranasal Oxytocin Administration
Intranasal administration of OT has been found to influence a variety of human behaviors. It is important to bear in mind that the original study demonstrating uptake of vasopressin into CSF found that peripheral vasopressin also increased, complicating interpretation of behavioral changes.
50 Prolonged increase in peripheral OT after intranasal administration has been demonstrated in humans.
52,53 However, a study in humans comparing the effects of intranasal and intravenous administration of vasopressin reported that only intranasal administration altered event-related potentials evoked by an auditory attention task.
51 Recently, a study in nonhuman primates confirmed both an increase in CSF levels of OT after intranasal administration and altered prosocial behaviors.
54 At present, route(s) of uptake into the CSF have not been determined, and penetration of neuropeptides into relevant brain areas after intranasal administration has not been experimentally verified.
20,55The ability of exogenous OT to modulate many aspects of social-emotional behavior is strongly supported by recent reviews.
21,24,25,49,55–59 Administration of OT can improve multiple measures related to social perception (e.g., faces versus houses, social versus nonsocial stimuli, biological versus nonbiological motion) without improving detection, suggesting that OT enhances a later stage in processing. More complex aspects of social cognition (e.g., emotion recognition and categorization, memory for socially-relevant emotional information, parent–infant interactions, trust-related behaviors) may also be enhanced. There is evidence that OT increases emotional empathy (affective sharing), but not cognitive empathy (mentalizing), indicating improved recognition of feelings, but perhaps not greater understanding of problems. OT can also increase behaviors that might be considered negative, such as envy and social aversion, possibly a reflection of enhanced noncooperation with individuals considered to be rivals (outgroup members). Variability in results across studies is likely partially due to the absence of standardization in key experimental methods related to OT delivery (e.g., drug formulation, amount and method of administration, and time between administration and behavioral measures).
60 Also, there is growing evidence that both situational factors (e.g., task difficulty, social context) and individual factors (e.g., gender, social proficiency, emotional sensitivity, coping style, personality traits, genetics) can influence the effect of OT administration.
25,49,58,61–64 The complex interplay between other hormonal and modulatory neurotransmitter systems and OT also complicates interpretation of findings.
Functional neuroimaging (primarily fMRI) has begun to shed light on the brain areas affected by intranasal administration of OT.
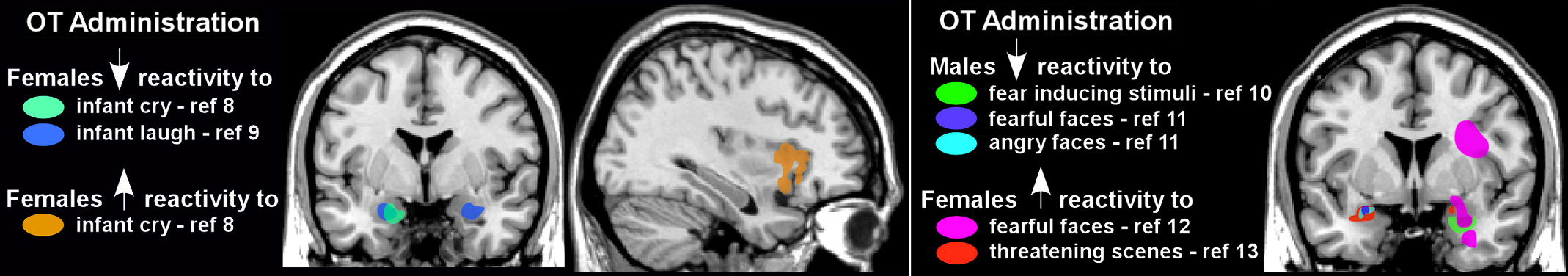
24,57,59 Altered amygdala reactivity to emotional stimuli has been the most common finding. For example, OT decreased amygdala reactivity in women exposed to either infant laughter or crying (
Figure 2) and in men exposed to negative emotional stimuli (e.g., fearful or angry faces, threatening scenes;
Figure 2).
8–11 These findings are consistent with proposed anxiolytic effects of OT. However, some of OT’s modulatory effects appear to be gender-specific, as amygdala activation was increased in most studies utilizing similar negative emotional stimuli in women (
Figure 2).
12,13,57,65 Several studies have also reported alterations in functional coupling between areas of the brain implicated in emotional processing (e.g., amygdala) and other brain regions after OT administration, although the specifics vary.
57,59,66,67Intranasal administration of OT has been considered as a potential treatment for several neuropsychiatric disorders, with indications of benefit for some conditions, although few randomized control trials (RCTs) have been reported.
19,25 Several studies in patients (mostly adult) with ASD indicate that OT administration improved measures related to social behavior (e.g., emotion recognition, gaze time on eye region, trust) and beneficial therapeutic effects have been noted in a few case reports.
19,24,25,48 Studies in patients with schizophrenia also have shown positive effects on measures related to common symptoms, including cognition, emotion recognition, and social perception.
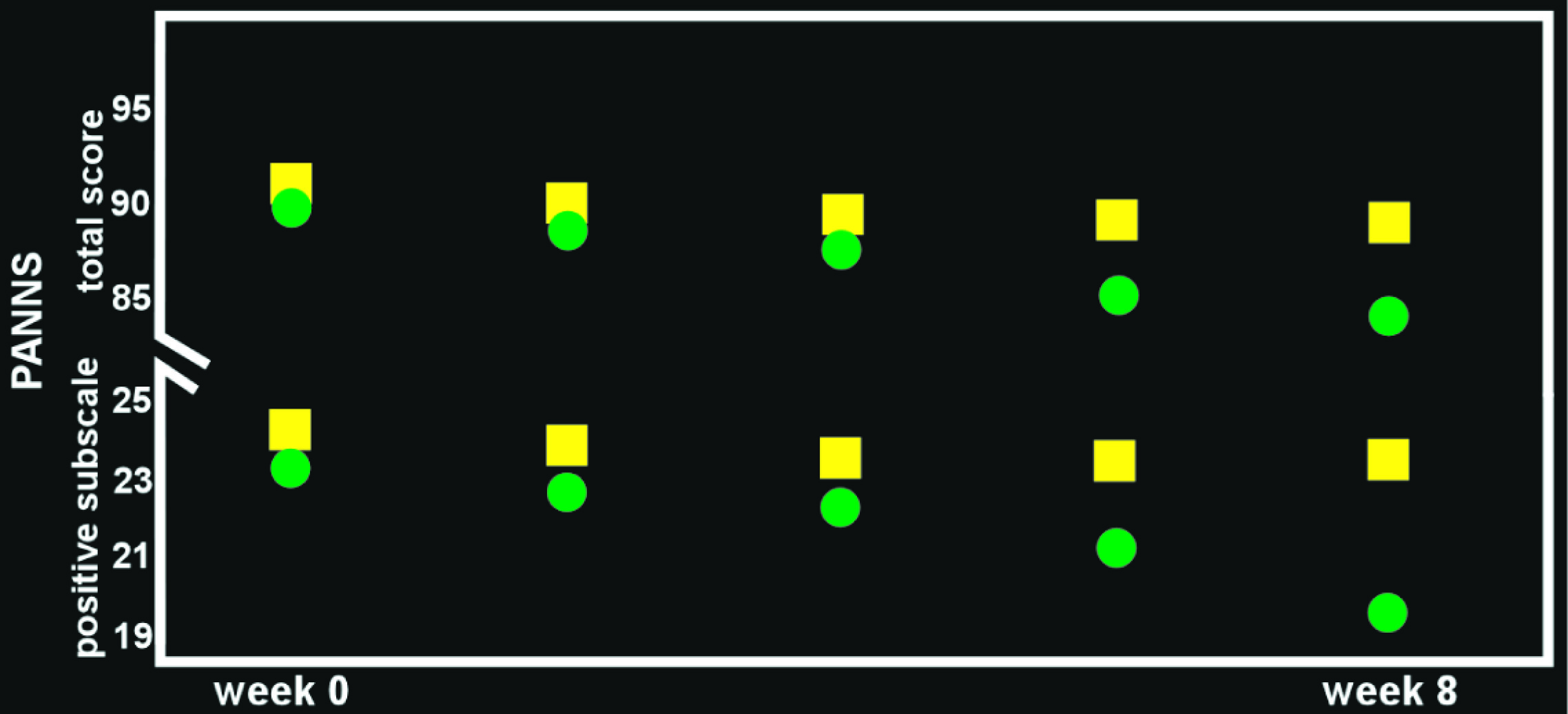
68–70 Several recent RCTs in patients with schizophrenia on stable doses of antipsychotic medication have reported that addition of OT had beneficial effects (e.g., reduced psychotic symptoms, improved social cognition;
Figure 3), although contrary results have also been reported.
14,47,69–76 The few studies in patients with borderline personality disorder (BPD) have been mixed, with one reporting a beneficial effect on emotional regulation and the other reporting reduction in trust and cooperation.
25 Findings in anxiety disorders have also been mixed. Although administration of OT has been shown to decrease fear-related amygdala activity in patients with generalized anxiety disorder, an RCT in patients with SAD found no main effect of OT administered before Sessions 2–5 of exposure therapy on objective outcome measures, although patients rated their appearance and performance as improved.
25 Two studies have reported symptom reduction in patients with posttraumatic stress disorder (PTSD) after a single dose of OT (e.g., diminished combat imagery-evoked physiological response, decreased intensity of anxiety) and one reported improved mood.
25 Two recent RCTs reported that the addition of 3,4-methylenedioxymethamphetamine (MDMA) to psychotherapy was associated with better outcomes (statistically significant in one) for patients with chronic, treatment-resistant PTSD, and MDMA-induced OT release has been implicated as an important mechanism of action.
77–79 It has also been suggested that administration of OT before psychotherapy may enable the patient to build trust in the therapist and strengthen engagement, thus facilitating the therapeutic process.
80,81