Cognitive loss is becoming increasingly prevalent in the United States. Approximately 4.7 million Americans currently have Alzheimer’s disease (AD), with a predicted growth to 13.8 million by 2050.
1 An additional 3%−22% of those over 60 years of age are thought to currently meet criteria for Mild Cognitive Impairment (MCI).
2–4 The early detection of MCI and dementia is critical, as studies have shown that early pharmacological intervention may delay the progression of AD.
5 Timely identification will become even more important once disease-modification treatments are available. Unfortunately, most cognitively impaired patients do not seek early medical attention, and physicians may not recognize subtle cognitive deficits during routine office visits. Patients typically present to their doctor 3−4 years after symptoms have begun.
6,7 Furthermore, even practitioners aware of cognitive complaints may not perform a cognitive assessment, make a diagnosis, or initiate medical interventions until a later, more progressed stage of the disease.
8–10 In fact, more than 40% of patients with mild dementia are not detected and diagnosed by their primary care physician.
11,12Methods
The research complied with the Declaration of Helsinki and was approved by the Ohio State University’s Biomedical Sciences Human Subject Institutional Review Board. Subjects over 50 years of age with sufficient vision and English literacy were recruited from a wide variety of community events, including senior centers, health fairs, educational talks to lay public, independent- and assisted-living facilities, and free memory screens through newspaper advertisements. Except at health-fair events, the audience was given a brief question-and-answer presentation on cognitive impairment and memory. The SAGE cognitive screening tool was given to all subjects meeting criteria and willing to take the test. No incentives were provided. We excluded individuals who had indicated to our written question that they had taken the SAGE previously. Subjects were usually tested in large groups except for those at health fairs or free memory screens, where they would come 1−6 at a time. Normally, we would have one or two administrators at these events to distribute, collect, and grade the SAGE on the spot. Subjects were provided their score and written information about the SAGE and were advised to show this to their physician for interpretation and potential further screening or evaluation based on their health history, if indicated. All were told that this test represented their baseline, to be compared with future rescreening by their physician. Individuals who wanted to discuss their results further were able to do so in a more private place.
Most settings were quiet when the individual took the test, but there was noise and other distractions at times, as may occur at health fairs and in community events. Cheating was minimized, as we ensured that those in close proximity had a different form of SAGE to reduce “wandering eyes.” Since this is a self-administered test, subjects could take as much time as they wished. For consistency, the same researcher scored all tests.
The SAGE can be divided into 6 different domains: Orientation (month+date+year), Language (verbal fluency+picture naming), Reasoning/Computation (abstraction+calculation), Visuospatial (three-dimensional construction+clock-drawing), Executive (modified Trails B+problem-solving), and Memory. Each domain is scored from 0 to 4 possible points, except the Memory domain, which is scored from 0 to 2 possible points. At the beginning of the test, there are non-scored questions about past history of strokes, family history of cognitive impairment, and current impairments in cognition and activities of daily living. These questions are designed to help the clinician to identify possible causes of cognitive decline. Patient age and educational level is reported on the test, which is designed to assist the clinician in interpretation of the score, as educational level (cognitive reserve) and age may affect the timing of the appearance of cognitive complaints. There are four slightly different versions of SAGE, designed to avoid practice effects and to prevent cheating when SAGE is given to large groups with individuals in close proximity. On the basis of our previous validity studies, as compared with neuropsychological testing, subjects with SAGE scores of 22 (maximum score) to 17 are likely to have normal cognition, whereas those with scores of 16 and 15 are likely to have MCI, and those with scores 14-or-less are likely to have dementia.
26 These historical cutoff values were used in this study to determine who had MCI and dementia.
Statistical Considerations
In this descriptive study, significance was determined by a two-tailed p value under 0.05. Summary statistics are reported as mean (± standard deviation [SD]). Bonferroni correction was incorporated in the examination of non-score items. Principal-components analysis was performed on all 6 domains of the SAGE.
27 Score distribution, reliability, and correlations between individual SAGE test items and total SAGE score were evaluated. We also studied the impact of education, age, gender, SAGE version (Test Forms 1, 2, 3, and 4), and race on the total SAGE score by use of a stepwise regression modeling approach in which the final model contained only significant (p <0.05) predictors. Pearson correlation was used to examine pairwise associations.
Results
A total of 1,047 individuals from 45 community events from March 2006 to September 2011 were screened for their cognitive abilities by use of the SAGE. Since there were so few non−African-American minority subjects (19 Asians, 4 Hispanic, 5 Other, 6 race unknown) these were excluded from the final analysis. Also excluded were those whose education level (N=24) or gender (N=23) data were not provided.
Table 1 lists the location and types of community events where the resultant 966 participants were evaluated. They were 71% women, 7.9% African-American, and 54.5% college graduates, with a mean age of 72.9 (8.7) years. On the basis of SAGE score, 71.6% were classified as Normal, 10.4% as MCI, and 18.0% as Dementia. The mean SAGE score was 17.8 (3.7).
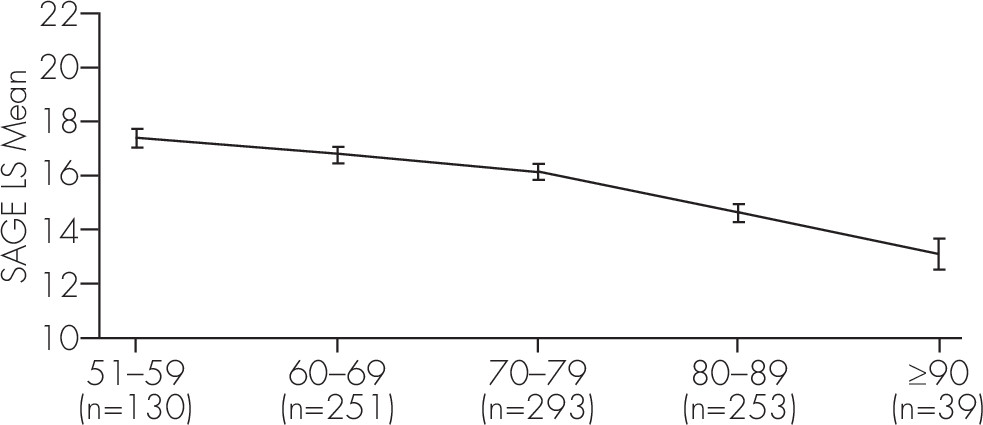
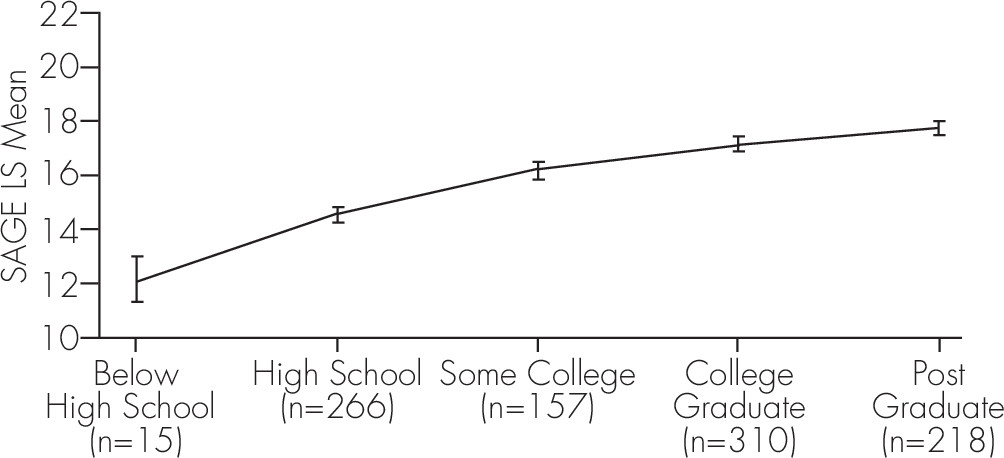
A stepwise regression analysis was carried out on those 966 subjects who had all the demographic information. As expected, older age (F[4, 956]=28.17; p <0.0001) and lower education level (F[4, 956]=43.06; p <0.0001) were both associated with lower total SAGE scores, as shown in
Figure 1 and
Figure 2. Gender and version of SAGE did not significantly affect total SAGE scores. The mean SAGE score for White participants was 18 and for African-Americans 16, however, the data were not balanced with respect to race frequency and age distribution; 92% of subjects were White, and many more African-Americans (mean age: 65.5 years) than Whites (mean age: 73.6 years) were younger. There was also a confounding effect due to the association between African-American race and lower educational achievement (mean education level was 13.9 years for African-Americans and 15.1 years for Whites). After controlling for age, education, and race, there was no difference between the mean total SAGE scores among the different screening locations and types of community events (
Table 1).
In order to examine collinearity issues between the 6 SAGE domains, we carried out a correlation analysis that yielded Pearson correlations in the range of 0.10 to 0.69, all statistically significant (because of the large sample size). There were low correlations between Orientation and other variables (<0.15) and moderate correlations between the rest (0.24–0.45), with the exception of Language and Visuospatial scores (0.69). Principal-component (PC) analyses based on the correlation matrix revealed that the first and second PCs are statistically significant, explaining 59% of the variability in the six-dimensional SAGE domain data. The remaining 4 PCs explained 41% of the variability. The weights for the domain scores for the first two PCs are shown in
Table 2. The first PC reflects the near-equal weights given to the scores of the first five domains and accounts for 42% of the variability in the data, and the second PC represents exclusively the Orientation domain, which accounts for the remaining 17%. Thus, the first PC values equally the performance in the Language, Reasoning/Computation, Visuospatial, Executive, and Memory dimensions. The correlation data in
Table 2 are consistent with the outcome of the PC analysis, with high and near-equal correlations for the same five domains to the total SAGE score. Both indicate that SAGE is an internally-consistent test that is very well balanced, with Language, Reasoning/Computation, Visuospatial, Executive, and Memory domains each contributing equally and similarly to the variability of the data. It suggests that no single domain is over- or under-represented in the scoring of this test. The Cronbach’s α for the six domains was 0.71.
We also evaluated the impact of the seven non-scored SAGE items on the total SAGE score, using analysis of covariance that controlled for age, education, and race. Upon utilizing Bonferroni correction with an overall α level of 0.05, only those subjects reporting a history of a stroke (F[1, 955]=10.3262; p=0.0014) had significantly lower SAGE scores (p <0.0071 based on Bonferroni correction of 0.5/7), with an average SAGE score reduction of 1.2 points. Those subjects reporting a balance problem (F[1, 943]=0.7425; p=0.2891), feelings of being sad or depressed (F[1, 946]=6.3945; p=0.0116), problems with memory or thinking (F[1, 946]=1.7561; p=0.1854), blood relatives with problems with memory or thinking (F[1, 928]=0.3143; p=0.5752), change in their personality (F[1, 917]=5.0032; p=0.0255), or difficulties doing everyday activities because of thinking problems (F[1, 940]=6.6085; p=0.0103) did not reach the threshold for having significantly lower SAGE scores.
Discussion
In the community settings described (
Table 1), SAGE, a brief, validated, self-administered cognitive assessment tool, identified 28.4% of those over 50 years old screened as having cognitive impairment. These are typical percentages that have been reported from other prevalence sources.
11,13Most individuals completed the SAGE within 10–15 minutes. The SAGE can be scored in seconds. One or two people can hand out, monitor, collect, grade, and provide test results with interpretation to the individuals very quickly. Thus, cognitive screening using SAGE is very practical, and it provided consistently reliable test results in small, medium, and large community group settings.
There are many excellent cognitive screening tools, but most are not self-administered.
28–39 Although web-based cognitive testing is becoming more popular to self-assess and monitor cognition, many cognitively impaired subjects shy away from being tested at all, and some on-line testing has shown poor validity as compared with pencil-and-paper screening.
40 The only self-administered tool studied in the community showed limited utility.
16 Although there is debate about whether proactive community-based screening is meaningful in predicting deficits upon further testing, there are reports showing positive correlation.
41 There is also some concern that cognitive screening may cause harm or undue anxiety to the subjects. This may be less of an issue with SAGE, as there is no administrator asking questions and no time limit to complete it. Also, non–self-administered examinations make it nearly impossible to rapidly screen large numbers of individuals in community settings. They are also impractical to be feasibly applied in many busy healthcare settings because they take up too much time and personnel resources. The SAGE provides an alternative so as to overcome these barriers to cognitive screening.
Most cognitive assessments of some breadth will recommend normalization of the test score based on the participant’s age and educational achievement. For the SAGE, based on our findings (
Figure 1 and
Figure 2), we recommend that 1 point should be added to scores when age is over 80, and 1 point should be added when education level is 12 years or less. Furthermore, since only 15 subjects self-reported less than 12 years of education, we are unable to make definitive conclusions for the meaning of SAGE scores for this group. A larger sample of people with education less than 12 years should be studied utilizing SAGE, particularly since this would be a group that both is likely to have some literacy problems with a written screening test, and may manifest with cognitive difficulties early (less cognitive reserve).
On average, African-Americans were 2 points lower than Whites for every education level except those with postgraduate degrees, where they performed better. Because of multiple confounding effects, we are unable to draw more definite conclusions regarding any race effect on SAGE scores.
The version of SAGE utilized did not significantly affect total SAGE scores. This is consistent with our previously published validity study.
26 Having four equivalent, interchangeable forms is particularly helpful, both to reduce learning effects of repeated administrations and to be able to rapidly screen large numbers of individuals at the same time.
Two major components were identified with principal-components analysis; one measuring a dimension including mostly the domains of language, reasoning/computation, visuospatial, executive, and memory, and the other measuring mostly the domain of orientation. Since the SAGE total score gives weight to both major principal components, we conclude that all the domains tested in SAGE are well represented in its total score.
In looking further at the correlations between SAGE Domain scores and its Total score (
Table 2), it is encouraging to see how well balanced the scoring is between the domains. The domains and questions in SAGE were chosen for their ability to be early predictors of MCI due to any condition and not necessarily to detect just pre-Alzheimer’s disease by over-representing the memory domain, as some cognitive tests do. Other degenerative dementias will have early and more pronounced deficits on executive (frontotemporal dementia), visuospatial (dementia with Lewy bodies), or language (primary progressive aphasias) domains. SAGE appears to provide similar weightings for each domain (other than orientation). The low correlation of the orientation domain to total SAGE score is likely related to the facts that less than 20% of our sample probably had dementia and that most normal individuals were able to be very accurate with the date. Nevertheless, orientation assessment is a very important domain that is well represented, but not overly represented, in the SAGE.
Answering affirmatively to any of the 7 non-scored SAGE questions leads to lower SAGE scores after adjustment for age, education, and race. However, only scores for subjects reporting a history of having either a major or minor/mini-stroke reached significance after a Bonferroni correction. For community screening, in addition to stroke, individual responses on these non-scored questions may provide etiological indications that could direct further evaluations.
There are significant limitations in this study. Only individuals able and willing to attend these community events in central Ohio and take SAGE were included in our sample. Although we were very diligent in identifying multiple sites and venues (
Table 1) for the recruitment and testing, these results should not be generalized to a population of all those over 50 years old. One-third of our subject recruitment came from community health fairs, and these subjects were perhaps less worried about memory. A small number were recruited from memory screening through newspaper advertisements and were probably more worried about their memory. There may be reasons, including cognitive concerns, that drive certain individuals to come to a lecture on memory or take the SAGE test at a health fair. That is probably why we had over 28% of subjects testing in the cognitively-impaired range on SAGE. We also had a well-educated sample that may be skewing the generalizability of our findings.
There are limitations with the SAGE instrument as a screening tool. Individuals must be literate and have adequate vision and writing skills to answer the questions. The SAGE can be administered to these individuals verbally, but then it loses its practicality. In community screenings, lower-scoring individuals may not follow through with their physician for formal evaluations. It may be worthwhile in these cases to ask permission to send results to their doctor or to talk to a spouse or family member, if any, who accompanies the person, to increase the likelihood of physician follow-up. When primary care physicians utilize SAGE screening, then formal testing can begin when their patient’s SAGE scores warrant. There will be false positive and false negative results based on validity testing showing sensitivity 79% and specificity 95%.
26 Assessment of memory is difficult in a self-administered test, and the memory domain in the SAGE only consists of 2 points maximum because of this. However, principal-components analysis and correlation data do seem to validate the memory question in contributing roughly equally with the other domains to the variability of the data. Finally, this study only examines SAGE as a screening tool, as no further testing was done to identify whether individuals scoring in a presumed MCI or dementia range had clinical evidence for the presence of those conditions.
The annual cost of dementia care has been estimated to be between $157 and $215 billion, resulting in a substantial financial burden on patients, families, and society.
42 Intervention earlier in the dementia course would likely affect the associated costs of caregiving and long-term care placement by leading to timelier pharmacological intervention.
43 Therefore, effective and efficient means for identifying community-residing individuals with cognitive impairment should be a priority.
Community screening using SAGE was found to be feasible, practical, reliable, and efficient in a variety of different venues. SAGE is able to rapidly screen large numbers of individuals in the community at the same time because of its self-administered feature and having four interchangeable forms that reduce the temptation of dishonesty. In our study, 28.4% of those screened were identified as having cognitive impairment, based on previously published standards for the SAGE test. Finding these individuals through screening can “start the conversation” about cognitive impairment with their primary care doctors and potentially lead to earlier evaluation, management, and treatments. It also potentially makes it easier to find research participants at the early and pre-dementia stages, to evaluate new therapies. Future studies need to compare SAGE with cognitive screening measures that require more administration time. We also need to better address minority populations and low-education cohorts by focusing screening in rural, minority, and underserved communities.