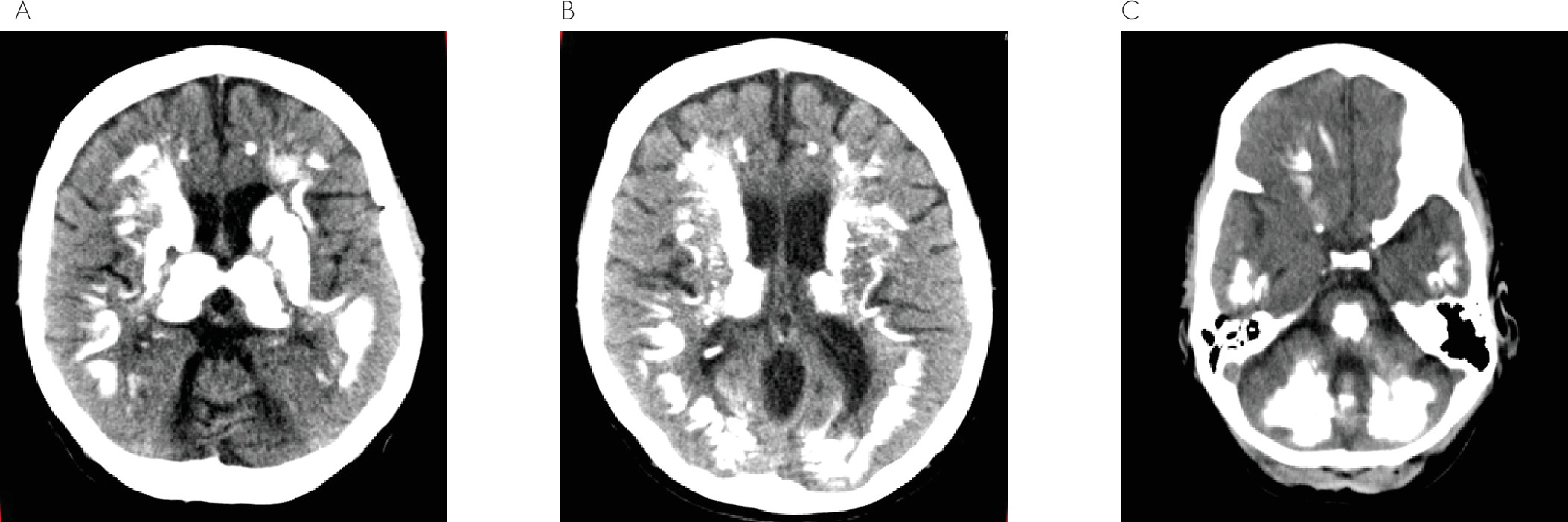
Fahr’s disease is a rare neurodegenerative disorder characterized by idiopathic bilateral basal ganglia calcifications, with a possible higher incidence in men.
1 The most common presentation in symptomatic patients is movement disorders, of which parkinsonism is predominant.
1 Other neurologic symptoms include cognitive impairment, cerebellar signs, speech disorder, pyramidal signs, psychiatric features, sensory changes, and gait disorders.
1 The transmission is most often autosomal dominant.
2 For years there has been inquisitiveness to comprehend the disease process, and researchers have extensively investigated images from autopsy specimens. Immunohistological studies of brain lesions in Fahr-type calcifications were performed by Fujita et al
3 for 19 patients and showed variable findings including diffuse neurofibrillary tangles with calcification, Alzheimer's disease, Pick's disease, progressive supranuclear palsy, and Parkinson's disease. Moreover, three different patterns of calcium deposition were observed: diffuse deposition within the tunica media of small- and medium-sized vessels, free spherical or lobulated concretions in the parenchyma, and rows of small calcospherites lying along capillaries.
3 Some radiological studies showed that, at times, the intensity of calcification may not correlate with neurological impairment.
4Certain gene mutations have been implicated for its possible etiology, and mutations in SLC20A2 [solute carrier family 20 (phosphate transporter), member 2] are considered one of the major causes of familial idiopathic basal ganglia calcification.
5,6 Other genetic mutations reported are PDGFRB [platelet-derived growth factor receptor, beta polypeptide] (especially seen in families with an absent SLC20A2 mutation) and chromosome 14q, 2q37 loci variations.
7–9 In another study, mutations in pantothenate kinase 2-related neurodegeneration were described and associated with idiopathic basal ganglia calcifications.
10The role of autoimmunity in Fahr’s disease is described very little in the literature. Sava et.al described a case of intracerebral symmetrical calcifications secondary to hypoparathyroidism. The patient’s autopsy of the parathyroid glands showed fibroadipose tissue, suggesting a remote autoimmune pathology of the parathyroid glands.
11 Morgante et al. hypothesized that gliovascular changes caused by cerebral inflammation may facilitate calcifications within the striopallidodentate system when there is a disturbance in calcium metabolism.
12 Another clinical case of a patient who presented with loss of consciousness and convulsions was reported by Arranz Perez et al., who described calcifications of the basal ganglia, hypocalcemia, and myocardiopathy of hypoparathyroidism, and attributed these findings to autoimmune polyendocrinopathy.
13 Fahr’s disease has also been described in association with idiopathic pulmonary hemosiderosis and primary hypoparathyroidism, which were considered related because of their autoimmune nature.
14 Similarly, the syndrome has been seen with pseudohypoparathyroidism and autoimmune hypothyroidism.
15