An “endophenotype” can be defined as a hereditary characteristic that is normally associated with a condition but is not a direct symptom of it.
1 This term has gained much attention in the past decade, particularly in the context of studying the genetic basis of neuropsychiatric disorders, including autism spectrum disorder (ASD). In part, this trend is driven by the extreme complexities of psychiatric genetics. Multiple genes are thought to underlie most mental illnesses. Moreover, there is a distal and convoluted connection between the genetic code and resulting phenotypes. These challenges motivate researchers to look for phenotypes that are more readily quantified and lie closer to the genetic basis of neuropsychiatric conditions. By examining simpler phenotypes, or endophenotypes, the hope is that susceptibility genes will be identified. Arguably, the endophenotypes of greatest utility are biological measures of neural substrates that are more proximal to susceptibility genes.
2Diffusion tensor imaging (DTI) is a type of structural MRI that enables in vivo assessment of white matter abnormalities by measuring fractional anisotropy (FA), a measure of fiber tract integrity derived from local water diffusion properties. Since the first DTI study was published a decade ago, dozens of studies have followed, which generally found reduced FA in children with ASD; however, only one study to date has reported on unaffected siblings (US) of individuals with ASD.
3 This study sought to corroborate their findings that diffusion imaging could identify a neuroendophenotype in US while more strictly excluding the broad autism phenotype in siblings.
4 We hypothesized that although the behavioral phenotype is completely absent in US, their brain phenotype is similar to that of children with ASD, albeit with less abnormality. On the basis of our previous work,
5 we predicted that ASD and US groups would exhibit greater abnormalities in the forceps minor and inferior fronto-occipital fasciculus compared with typically developing (TD) children.
Methods
Patients
The DTI data collected were part of a larger set for functional MRI studies in our group. No significant between-group differences were found for age, gender, or handedness (p≥0.05;
Table 1). The study included a total of 50 participants: 19 with ASD, 20 US, and 11 TD controls. All participants were screened for neurological disease or insult that could be related to abnormal brain development, including seizures and head injury with loss of consciousness.
For the ASD group, diagnosis was based on parental information, clinical history according to criteria in
DSM-IV-TR,
6 and evaluation by licensed psychologists with expertise in autism. The evaluation consisted of gold standard assessments, including the Autism Diagnostic Interview–Revised and the Autism Diagnostic Observation Schedule administered by a licensed psychologist who is research reliable on both assessments.
7,8 All ASD participants were high functioning, with cognitive abilities group matched to TD participants using the Differential Ability Scale–II.
9For the US group, the ASD diagnosis of a sibling was confirmed using
DSM-IV-TR.
6 It was not a criterion that the affected sibling participated in this study, although some sibling pairs were used. An important characteristic of this study is that all US participants were screened and excluded for the broad autism phenotype using the Social Responsiveness Scale (<76), and US and TD participants were group matched based on their scale scores.
10 In addition, a semistructured clinical interview was conducted with US participants to further confirm the absence of autistic traits and to rule out any history of other neurological or psychiatric conditions. This strict exclusion criterion ensures that any identified differences in white matter structure between US and TD groups are not the result of use-dependent processes and are not reflective of subtle differences in social ability. Most US participants’ cognitive abilities were evaluated using the Wechsler Abbreviated Scale of Intelligence.
11TD participants were screened for the broad autism phenotype with the same semistructured clinical interview and Social Responsiveness Scale cutoff score as US participants. In addition, TD participants were screened for history of ASD in first- or second-degree relatives. All group characteristics along with relevant statistics are summarized in
Table 1. The Yale Human Investigation Committee approved this study. Informed consent was obtained from a parent/guardian and each participant provided verbal assent.
Neuroimaging
Three-tesla MRI was performed with the following DTI parameters: directions=30, b0=5, repetition time=6200 ms, echo time=85 ms, field of view=240×240 mm2, thickness=2.5 mm (isotropic), number of slices=55, averages=3, and total scan time=11 minutes. Whole-brain T1-weighted MRI was performed using a sagittal 1-mm3 magnetization-prepared rapid gradient echo sequence. All imaging was performed in the same session after completing mock MRI session(s).
Quality Assurance
The quality of diffusion MRI data was ensured through a multistep procedure that addressed eddy current distortion, head motion, and signal dropout. First, diffusion-weighted images were corrected for eddy current distortion and head motion. Second, all volumes (i.e., diffusion directions) were individually inspected for head motion and signal dropout by an experienced rater (R.J.J.) who performed DTI analyses in previous studies.
5,28 Volumes with excessive motion or dropout were spliced out and excluded from the analysis. If more than six volumes required exclusion, then the entire run was dropped from the analysis. Using this method, one participant from the TD group and one participant from the ASD group were excluded from the analysis; no participants were excluded from the US group. Next, multiple runs (per subject) of diffusion-weighted data were averaged to improve the signal-to-noise ratio. FA maps were individually assessed to confirm absence of artifact or distortion.
Data Analysis
All data processing and local diffusion modeling were conducted using the FMRIB Software Library (FSL) (fsl.fmrib.ox.ac.uk/fsl/). The program was used to generate a mask to separate brain areas from nonbrain areas. Using the FMRIB’s Diffusion Toolbox (FDT), a diffusion tensor model was created and FA, axial diffusivity, and radial diffusivity were computed for each voxel.
Three group-wise comparisons were made: 1) ASD versus TD, 2) US versus TD, and 3) ASD versus US. Voxel-wise analysis of multisubject diffusion data was conducted using tract-based spatial statistics (TBSS). The procedure consisted of the following steps: 1) application of nonlinear registration so that all images were in Montreal Neurological Institute space, 2) creation of a mean image, 3) skeletonization of the mean image, 4) projection of all subjects’ diffusion imaging data onto the mean skeleton, and 5) submission of the four-dimensional–projected data for statistical testing. Areas of significant difference were identified (p<0.05 corrected for multiple comparisons across space using threshold-free cluster enhancement or TFCE). Voxels with significant differences in FA values were labeled as “affected voxels,” and only these voxels were used in the comparison of FA, axial diffusivity, and radial diffusivity between groups. Using the T1-weighted MRI data, intracranial volume was calculated using the FreeSurfer image analysis suite (surfer.nmr.mgh.harvard.edu/).
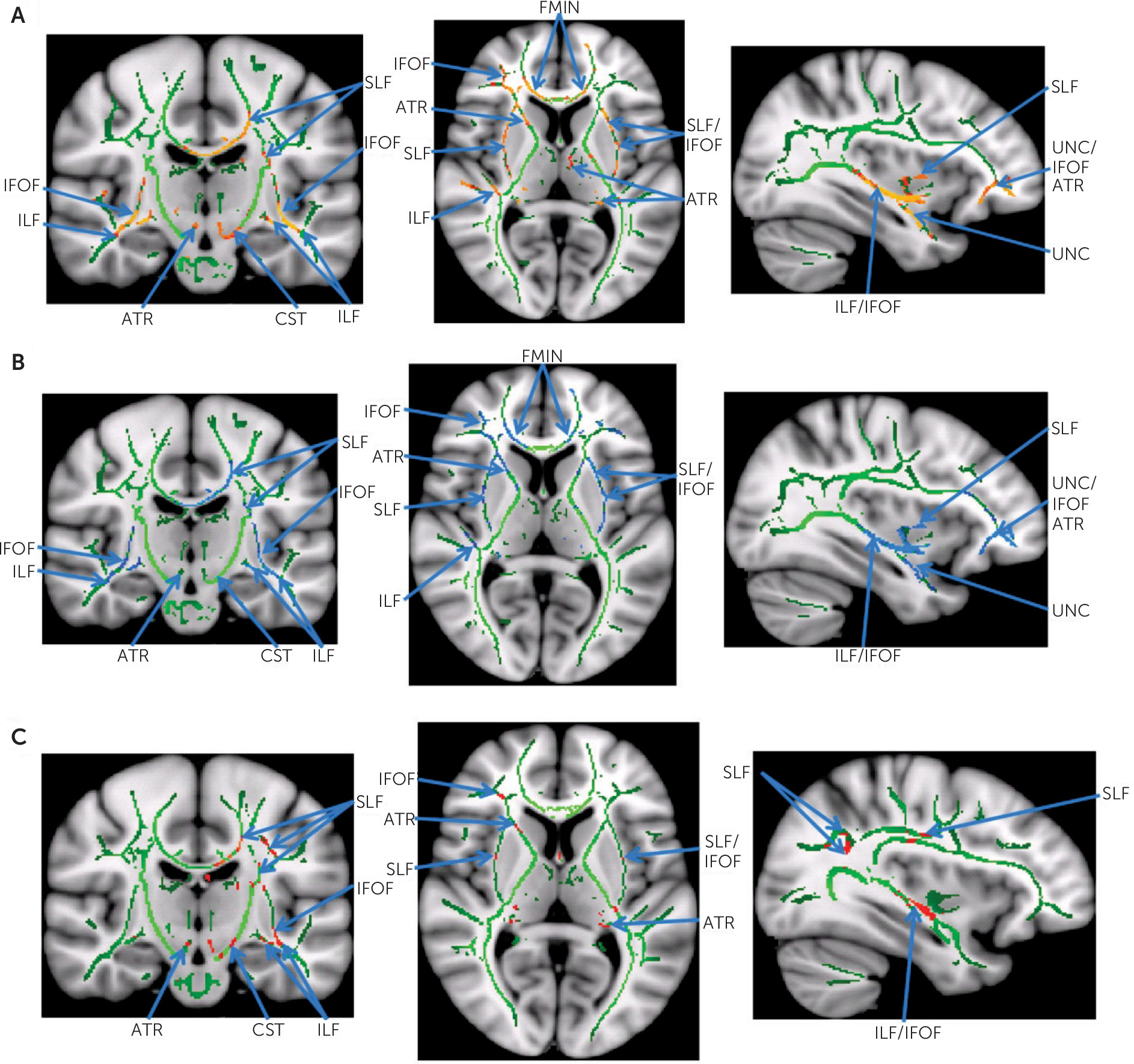
Affected white matter structures (fiber tracts) were identified using the Johns Hopkins University White Matter Tractography Atlas. This atlas was not only useful for identifying potentially affected fiber tracts, but it was also useful for more precise characterization of potential abnormality by quantifying affected voxels based on their tract labels. The Montreal Neurological Institute coordinates of all affected and unaffected voxels were captured and intersected with the aforementioned atlas. These voxels were assigned to atlas labels linking them to their respective association, commissural, or projection tracts. For each participant, affected voxels were grouped by label with their corresponding metrics (FA, axial diffusivity, or radial diffusivity), and counts were generated for each fiber tract. Summary statistics for FA, axial diffusivity, and radial diffusivity were computed for each fiber tract, first by individual and then by group. Percent abnormality was calculated for each tract by dividing the number of affected voxels by the total number of voxels compared. This represents the degree of white matter abnormality by tract.
Post-hoc Pearson's correlation analyses were performed between mean FA within affected fibers and SRS total scores. Where appropriate, Bonferroni correction implemented for multiple comparisons.
Discussion
As the term
broad autism phenotype implies, the symptoms or behavioral phenotype of ASD lie on a continuum.
4 In this study, both US and TD groups lie on the unaffected end of the spectrum, which was ensured by strictly excluding participants with a broad autism phenotype. This study explored whether US, who show no signs of autism, exhibit an intermediate neural phenotype based on their genetic liability for ASD. The primary goal of this study was to detect the presence of this neuroendophenotype in US. Notably, the presence of neural abnormalities in US (matched to TD on behavioral phenotype) would suggest that this is an endophenotype and not a function of experience-dependent processes.
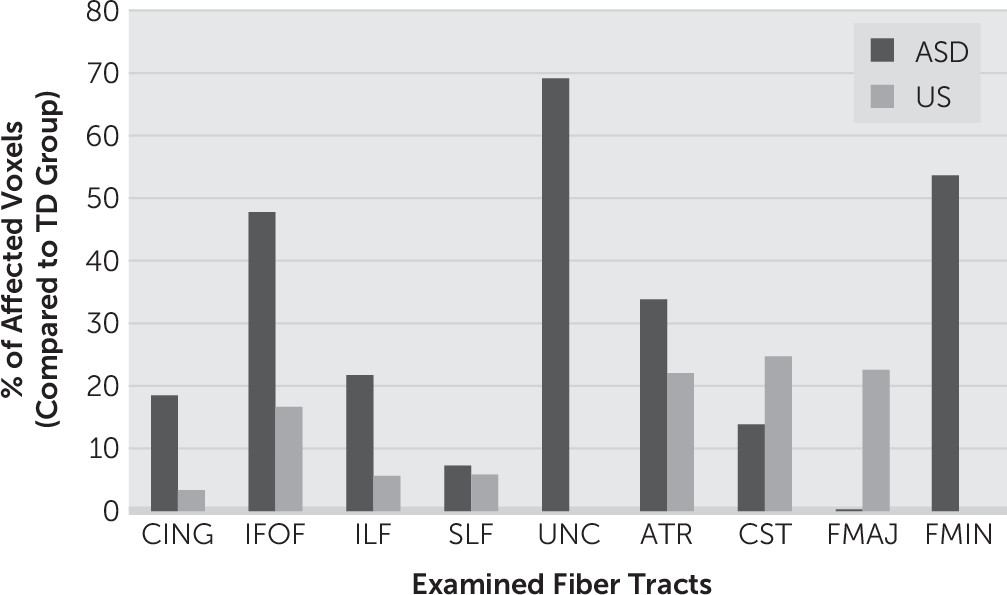
As presented above, there were no significant abnormalities when comparing the ASD group with the US group, suggesting that the neural phenotypes are similar. Interestingly, there are significant differences in multiple diffusion measures when comparing the ASD group with TD participants and the US group with TD participants. The differences are much greater in the former than in the latter; moreover, some of these differences do not overlap. That is, the difference between US and TD groups is not simply a milder version of the difference between ASD and TD participants (
Figure 2). These results suggest a neuroendophenotype that superficially resembles probands, although there are key differences in the severity and distribution of these abnormalities.
The apparent sparing of the uncinate fasciculus and forceps minor in the US group is remarkable (
Figure 2,
Table 3), especially given the observation that these two fiber tracts were the most severely affected in the ASD group. The importance of the uncinate fasciculus, an intrahemispheric association tract of the frontal and temporal lobes, has already been recognized in the pathophysiology of ASD.
12 The uncinate plays a role in the mirror neuron system, a complex of neurons repeatedly reported to be aberrant in ASD.
13–15 The uncinate fasciculus also includes projections into the cortical nuclei of the amygdala, a limbic structure involved in human social interactions, emotion processing, and pathophysiology of ASD.
16–18 Functional MRI studies in ASD have repeatedly revealed aberrant functional connectivity between the frontal and temporal lobes.
19The prominent abnormalities of diffusion parameters in the forceps minor, part of the anterior aspect of the corpus callosum, are in line with findings from a previous meta-analysis of MRI studies that focused on corpus callosum size and demonstrated a significantly smaller corpus callosum in ASD.
20 Disruption of the corpus callosum, a topographically organized interhemispheric structure, may contribute many of the sensory, cognitive, and behavioral symptoms observed in individuals with ASD.
21 Individuals with primary agenesis of the corpus callosum consistently exhibit impaired social skills and poor personal insight.
22 In one study, 8.5% of individuals with agenesis of the corpus callosum carried an ASD diagnosis.
23 In addition, previous functional MRI studies reported interhemispheric disconnectivity, suggesting corpus callosum involvement in ASD.
24,25The qualitative pattern of tract abnormality seen in the US group is congruent with the clinical differences in behavioral phenotype between the ASD and broad autism phenotype–negative US groups. First, the US group displayed a much lower level of tract abnormality in general, with maximum percent abnormality of any tract being 25% and 70% in the US and ASD groups, respectively (
Figure 2). Second, those tracts most affected in USs correspond to projection tracts, which have no clear role in social information processing. What the ASD and US groups have in common are abnormal diffusion measurements, which may represent general impairments in white matter integrity. Differences in both the severity and distribution of these abnormalities may account for the difference in behavioral phenotype. However, it is premature to state that the aforementioned tracts differentiate ASD and US neural phenotypes until this can be confirmed in much larger studies.
These findings should be interpreted within the context of important study limitations. Participants were selected from data collected for previous functional MRI studies by our group. Because this was a retrospective analysis, we were not able to precisely match the groups on age and gender. Given the known changes of FA with age, our sample consisted of individuals within a limited age range, well past the age of diagnosis, and during a period of relative trait stability.
26,27 Although there were no significant between-group differences, we cannot exclude the possibility of developmental effects on our groups or the potential for gender discrepancies in white matter development. In addition, the majority of the US participants received the Wechsler Abbreviated Scale of Intelligence rather than the Differential Ability Scale, excluding the ability to directly match on cognitive abilities. We do not expect significant effects of IQ given that all group mean scores were within the average range. Findings reported here need replication with a larger population and greater TD sample size. Finally, the functional consequences of white matter abnormalities in US remain unknown without more detailed neuropsychological testing.
Overall, the intermediate neural phenotype in US demonstrates a milder pattern of white matter abnormalities with differences in which specific tracts are affected (
Figure 2). In agreement with recently reviewed DTI findings, this study provides further evidence of global abnormalities in long-range connectivity in ASD involving association, commissural, and projection fibers.
28 In addition, there is some suggestion of different brain anomalies in ASD involving the uncinate and forceps minor. The presence of significant differences only in the ASD group compared with TD participants and the US group compared with TD participants (and not ASD compared with US) suggests that the US intermediate brain phenotype is likely more similar to ASD, although the US behavioral phenotype is similar to the TD group. The finding that white matter tract abnormalities are present in US participants without the broad autism phenotype suggests that these findings are true endophenotypes and not the product of a use-dependent process.