Although epilepsy has traditionally been thought of as a late manifestation of AD severity and disease progression,
4–6 recent work revealed that epilepsy could precede the onset of cognitive symptoms in AD by a few years in a subset of patients.
7,8 In general, studies examining the co-occurrence of seizures and dementia suffer from a number of methodological flaws, including unclear reporting of EEG abnormalities, analysis of single time points, and limited descriptions of seizure semiology and seizure circumstances.
9 With the aging of the population, the incidence of dementia is expected to increase
10 and the incidence of seizures and its associated costs will also rise. Seizures also seem to accelerate cell death and cognitive decline in dementia.
11 Thus, there is a need for better understanding of the clinical interaction between dementia and seizures.
In this study, we aimed to analyze the occurrence of unprovoked seizures in patients with dementia in relationship to the onset of their cognitive symptoms and their dementia diagnosis. We also aimed to characterize the seizure semiologies, EEG findings, and medication responses in this patient population and to compare patients with early-onset and late-onset dementia.
Methods
A retrospective chart review covering a 10-year period (2004–2014) of outpatient and inpatient records was performed using a research patient data registry tool
12 relying on ICD-9 diagnosis codes consistent with epilepsy (345.x) and those with dementia or mild cognitive impairment (331.x). The registry allows the search of patient data at Partners HealthCare hospitals including Massachusetts General Hospital, Brigham and Women’s Hospital, Newton-Wellesley Hospital, and Faulkner Hospital. This study was approved by the Partners HealthCare institutional review board.
Exclusion criteria included the presence of cortical structural lesions on neuroimaging, provoking factors, infectious or paraneoplastic syndromes, seizure onset >5 years before development of cognitive symptoms, seizure semiology limited to myoclonus, and a history of developmental delay. Patients who were diagnosed with mild cognitive impairment and in whom dementia did not develop during the follow-up period were also excluded to confirm an underlying neurodegenerative condition and exclude patients who may have had medication-induced side effects.
Only patients with unprovoked seizures
13 were included in the analysis. Seizures were classified according to the 2010 International League Against Epilepsy classification.
14 Patients also had to fulfill criteria for dementia
15 at some point during the follow-up period. The dementia was further subclassified into AD,
15 dementia with Lewy bodies,
16 primary progressive aphasia,
17 behavioral variant frontotemporal dementia,
18 and vascular dementia.
19 The onset of cognitive symptoms reported by either the patient or caregivers as well as the date of the dementia diagnosis by a physician were noted. The duration of the follow-up was calculated as the time of evaluation at the onset of cognitive symptoms or seizures (whichever came first) until the last documented follow-up encounter.
Other clinical data included the patient’s educational level and psychiatric comorbidities at the time of seizure onset. The patient’s Mini-Mental State Examination
20 closest to the time of seizure onset was also recorded.
Epilepsy and EEG Data
The patient’s first seizure semiology as well as all seizure semiologies and frequency during the follow-up period were noted. For patients observed >1 year after seizure onset, a definition of medically refractory epilepsy was given if the patient continued to have seizures despite an adequate trial of two antiseizure medications.
21EEG findings at the time of first EEG and during the follow-up period were also assessed.
Statistical Analysis
Patients with early-onset (age <65 years) versus late-onset epilepsy were then compared using the Fisher exact test or Wilcoxon rank sum test when appropriate, with a p value <0.05 set for statistical significance. A logistic regression analysis was then performed to determine predictors of epileptiform abnormalities on the first EEG.
Results
A total of 879 patients were identified. Of these, 802 patients were excluded: 353 (44.0%) because of structural lesions on neuroimaging or provoked seizures; 350 (43.6%) because of insufficient details to justify the diagnosis of seizures or dementia; 59 (7.4%) because of developmental delay, of whom 19 had Down’s syndrome; 39 (4.8%) because of remote seizures; and one patient because of myoclonus as the sole seizure semiology (0.1%).
A total of 77 patients fulfilled the study criteria (
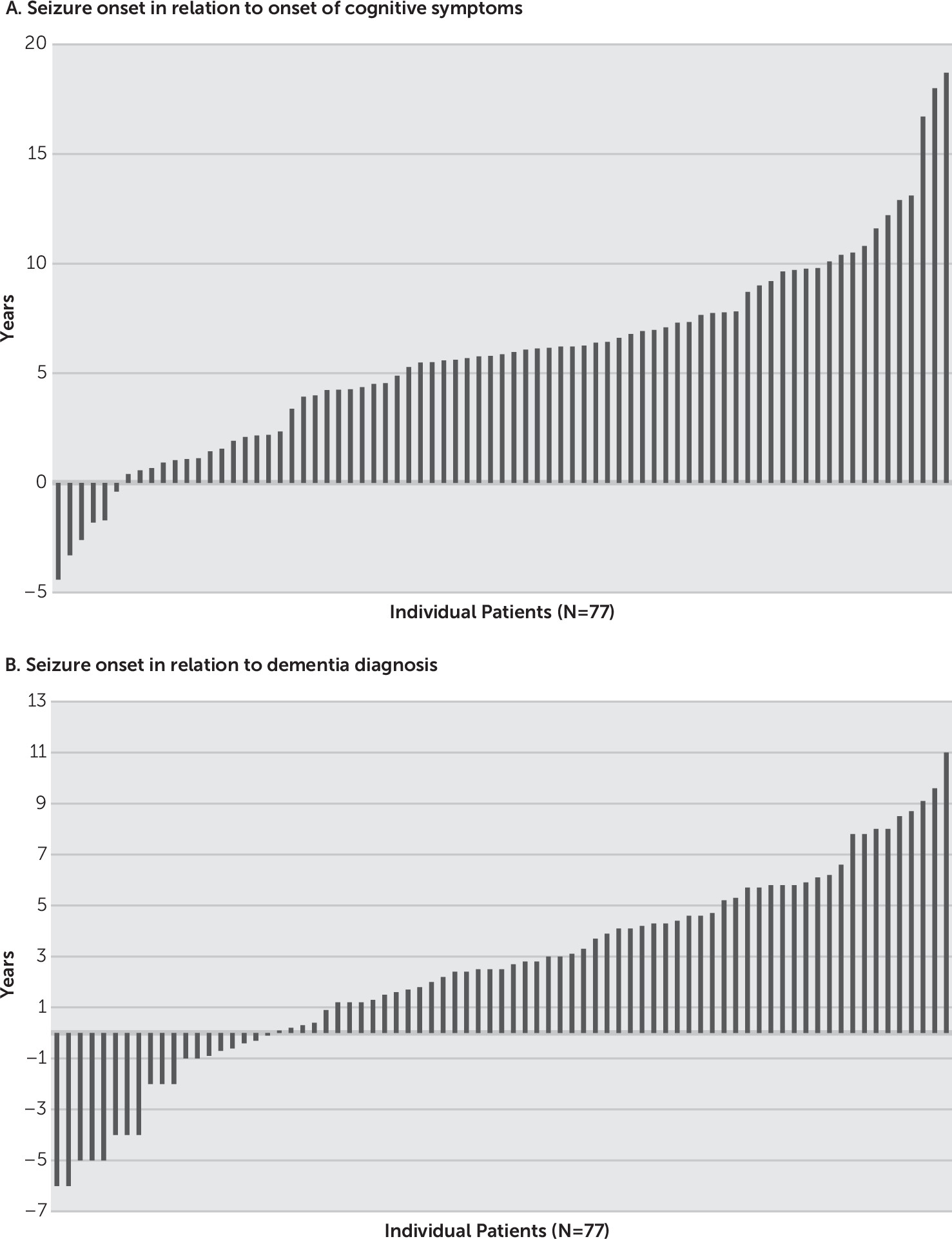
Table 1). Of these patients, 37 (48%) were evaluated in a dementia clinic, 8 (10%) in an epilepsy clinic, 13 (17%) in an epilepsy clinic and a dementia clinic, and 19 (25%) in a general neurology clinic. They were followed for a period of 6.6 years (range=0.5–21.9 years) overall and 4.0 years (range=0.1–17.5 years) after their first seizure. Average age at onset of cognitive symptoms was 68.1 years (range=34.0–86.0 years), and average age at seizure onset was 73.9 years (range=43.6–93.6 years). Seizures either preceded or followed the cognitive symptoms (range=4.3 years before and 18.7 years after) or dementia (range=6.4 years before, 10.9 years after) (
Figure 1). In 13% of patients, the seizures preceded cognitive symptoms or occurred within 1 year of symptom onset; average age at onset was 64.0 years (range=53.6–71.2 years).
In patients with non-AD dementia, seizures either preceded or followed the cognitive symptoms by 4.5 years (range=−3.3 to 13.1 years). At the time of seizure onset, four patients (5%) carried a diagnosis of a mood disorder and an additional four patients (5%) carried a diagnosis of an anxiety disorder. The Mini-Mental State Examination score closest to the time of seizure onset was available for 68 patients: <18 points in 38.3%, 18–24 points in 39.7%, and >24–30 points in 22.0%.
The final diagnosis was possible AD (13%), probable mixed AD with vascular dementia (5%), probable AD (65%), definite autopsy-proven AD (5%), dementia with Lewy bodies (5%), probable behavioral variant frontotemporal dementia (4%), vascular dementia (1%), and nonfluent primary progressive aphasia (1%). One patient had a known presenelin-1 mutation, and another patient had a microtubule-associated protein tau or MAPT mutation. Patients with Parkinson’s dementia and Huntington’s disease fulfilling inclusion criteria were not identified in the cohort.
The initial seizure semiology consisted of focal seizures without loss of consciousness in five patients (7%; two patients with an affective component, one patient with aphasia, one patient with epilepsia partialis continua of the right arm, and one patient with right arm clonic movements), focal seizures with loss of consciousness in 40 patients (53%), and generalized tonic-clonic seizures in 30 patients (40%).
During the disease course, 43 patients (57%) went on to have generalized tonic-clonic seizures, and six patients (8%) experienced myoclonus. Eleven patients (14%) reported a history of mild traumatic brain injury during their lifetime, and one patient had a history of encephalitis as a child. A family history of dementia was noted in 24 patients (31%), whereas a family history of epilepsy was reported in three patients (4%). None of the patients had a history of febrile seizures. The first EEG was performed within 1 week of seizure onset in 59% of the patients, with findings summarized in
Table 1.
Of the 17 patients with focal epileptiform abnormalities, seven had more than one spike population. Of note, one patient manifested nonconvulsive status epilepticus, and one patient exhibited epilepsia partialis continua.
At last follow-up, 12% of patients had refractory epilepsy. Levetiracetam was the most commonly used antiseizure medication (
Table 2).
When comparing early-onset (age <65 years) with late-onset dementia, we noted no statistical differences between the clinical and neurophysiologic characteristics (
Table 3).
Logistic regression analysis did not find any predictors of epileptiform abnormalities on the first EEG.
Discussion
Our study describing patients with unprovoked seizures and a diagnosis of dementia highlights the fact that seizures may precede or coincide with the presence of cognitive symptoms in neurodegenerative diseases. The majority of the seizures were controlled with medications. Epileptiform abnormalities were only noted in 36% of the patients, despite repeated EEGs to try to capture neurophysiologic abnormalities.
Patients with underlying dementia have a higher risk for the development of seizures, especially if the dementia is that of AD.
22 In an analysis of 83 patients with autopsy-proven AD, eight were found to have a history of seizures an average of 6.6 years after cognitive symptoms, whereas 10 patients had isolated myoclonus.
2 In their study quoting the highest seizure prevalence in dementia, Risse et al.
23 prospectively observed a cohort of 28 patients with dementia admitted to an inpatient research unit. They documented convulsive seizures in 64% of patients with AD and in 17% of patients without AD. The seizures occurred at a mean of 6.5 years after dementia onset and 2 years before death. On the other hand, Romanelli et al.
5 described a cohort of 44 patients with AD in which seven patients experienced convulsive seizures in the latter stages of their disease, prompting these authors to conclude that focal seizures and seizures during mild dementia should prompt a search for a diagnosis different from dementia.
Recent studies identified seizures across all disease stages. For patients enrolled in clinical trials with mild to moderate AD, a seizure incidence of 484 per 100,000 person-years was identified.
4In addition, Vossel et al.
8 analyzed 54 patients with amnestic mild cognitive impairment, AD, or subclinical epileptiform abnormalities and compared them with control patients with mild cognitive impairment or AD without epilepsy. The study group had an earlier onset of cognitive decline compared with the control group without epilepsy. A subset of these patients had the onset of seizures up to 10 years before the development of cognitive decline. The seizure semiologies in their cohort were quite diverse and included isolated experiential auras, focal seizures with loss of consciousness, and generalized clonic-tonic seizures. In a study of a more heterogeneous sample of patients with dementia, Rao et al.
24 described the co-occurrence of seizures in 39 patients with mild cognitive impairment, AD, vascular dementia, and dementia with Lewy bodies with focal seizures as the predominant seizure semiology.
In this study, we identified unprovoked seizures predominantly in patients with AD (83%) or AD with vascular dementia (5%) but also in patients with vascular dementia (1%), dementia with Lewy bodies (5%), primary progressive aphasia (1%), and behavioral variant frontotemporal dementia (4%). A significant proportion of patients (44%) were excluded from the study because of underlying structural abnormalities or provoked seizures. This trend is to be expected because structural lesions, especially those from cerebrovascular disease, tend to be more common in patients aged >65 years and account for the main identified seizure etiology.
2 Two percent of patients were also excluded because of a diagnosis of Down’s syndrome. Future analysis of this particular subpopulation of patients with dementia and seizures is of interest because of the high prevalence of AD pathology after age 40 years and new-onset epilepsy.
25A subset of patients (13%) had the occurrence of their seizures either preceding or occurring within 1 year of their cognitive symptoms regardless of the underlying pathology. Unlike earlier reports, our study results show varied seizure semiology with focal dyscognitive seizures, isolated auras, or generalized tonic-clonic seizures. The generalized tonic-clonic seizures are usually the main semiology that leads patients to seek medical attention. It remains unclear how common dyscognitive seizures are in patients with dementia.
Given these results, we propose considering the onset of a neurodegenerative disorder in the differential diagnosis of unprovoked seizures in patients with cognitive deficits. We cannot, however, exclude the possibility that the occurrence of the seizures and dementia may have been unrelated in certain patients. Subtle malformations of cortical development, for example, may be missed on neuroimaging but rarely cause seizures after age 50 years. We also tried to minimize the possibility of coincidental diagnoses by only including patients with seizures within 5 years of cognitive symptom onset.
Although previous studies noted that early age of dementia onset and severity of dementia were the most significant predictors for seizure occurrence,
1,4 our study did not find significant statistical differences in seizure characteristics between cases of early-onset and late-onset dementia.
Animal models have highlighted the interaction between seizures and neurodegeneration. In animal models of AD, hippocampal reorganization, hypersynchronization, and subclinical seizures are seen.
11,26 One theory advances that amyloid beta causes GABAergic sprouting, deficits in synaptic plasticity, and an imbalance in excitatory and inhibitory connections.
26 Amyloid beta also interacts with tau to cause neuronal hyperexcitability. It is also known that the reduction of tau reduces spontaneous epileptiform activity as well as lessens the severity of seizures.
27,28 The progressive accumulation of abnormal proteins in the brains of patients with neurodegenerative diseases could be a key component in the development of seizures in this patient population, with seizures being one of the presenting features of the disease preceding the cognitive impairment. Moreover, the onset of the neurodegenerative disorder could represent a “second hit” in already susceptible individuals with previous risk factors such as a family history of epilepsy, mild traumatic brain injury, or encephalitis.
Studies describing EEG features of patients with dementia and seizures are rare. Vossel et al.
8 published the largest cohort and found temporal epileptiform abnormalities to be the commonest neurophysiologic finding, although frontal and generalized discharges were also seen. These findings were unsurprising, considering that the temporal lobes are common sites of AD pathology and that AD and temporal lobe epilepsy share a number of common features.
29 These results were consistent with our study results, in which temporal and frontotemporal abnormalities predominated. Interestingly, of the patients with focal epileptiform abnormalities, 41% had more than one spike population, indicating that a wide irritative zone may possibly be the result of diffuse underlying pathology.
Meanwhile, the onset of generalized discharges could represent bilateral network hypersynchrony.
Although the incidence of epileptiform abnormalities was only at 36% despite repeated EEGs in some patients, we found it to lie in the expected range of EEG abnormalities in elderly patients.
30 It is unclear whether prolonged EEG monitoring in this patient population could lead to a higher yield.
In our cohort, seizures were relatively easy to control with medications, and only 12% of patients were medically refractory. At last follow-up, 54% of patients were maintained with levetiracetam. Of note, seizures are easier to control in elderly patients,
31 although one has to be cautious as to the actual determination of seizure frequency in this patient population because of the subtle seizure semiologies and the reliance of the clinician on the caregiver’s report. In a randomized trial of levetiracetam, phenobarbital, and lamotrigine in patients with AD, 58%−71% of the patients had >50% seizure reduction during a 1-year period.
32 Levetiracetam had the most favorable profile on cognition, although lamotrigine showed the most positive effect on mood. Given these results, we highly recommend taking into consideration the elderly adult’s medical and neuropsychiatric comorbidities as well as the treatment effect on mood and cognition while choosing the right medication to achieve seizure control.
Our study has a number of limitations, with the most constraining being its retrospective chart review nature. In addition, we note that patients were seen in different clinics and with a variable follow-up. Serial cognitive testing was not performed or documented in all cases and, at times, such testing was unavailable around the time of the seizure. Furthermore, EEG data were not available on all patients and were limited to scalp electrodes; thus, findings have to be interpreted in the context of the limitations of the scalp EEG. Data from other more invasive electrodes, such as depth electrodes or nasopharyngeal/sphenoidal/foramen ovale, were not available. Finally, the cohort was identified using the ICD-9 coding system; thus, patients who were not properly coded would have been missed.