Disorders of sleep have long been intertwined with neuropsychiatric conditions. The DSM-5 acknowledges a bidirectional relationship.
22 Once thought to be relatively rare, there is increasing evidence that obstructive sleep apnea (OSA) is both common and associated with significant medical and psychiatric comorbidities.
2,6,23 Risk factors for OSA include obesity, smoking, male gender and greater age.
10,11,23 There is an almost eightfold increase in prevalence of OSA in military veterans with combat exposure and a higher prevalence among patients with posttraumatic stress disorder (PTSD).
9,11 This condition has also been implicated as a risk factor for aggravation of epileptic seizures and development of dementia in older adults.
23 OSA with excessive daytime sleepiness (OSA syndrome) is associated with impaired occupational health (increased absenteeism and days sick) and lower job productivity as well as increased risk of work-related accidents.
24,25 The increasing prevalence and strong association between presence of OSA and both medical and psychiatric conditions makes it essential for clinicians to better understand this debilitating yet treatable condition.
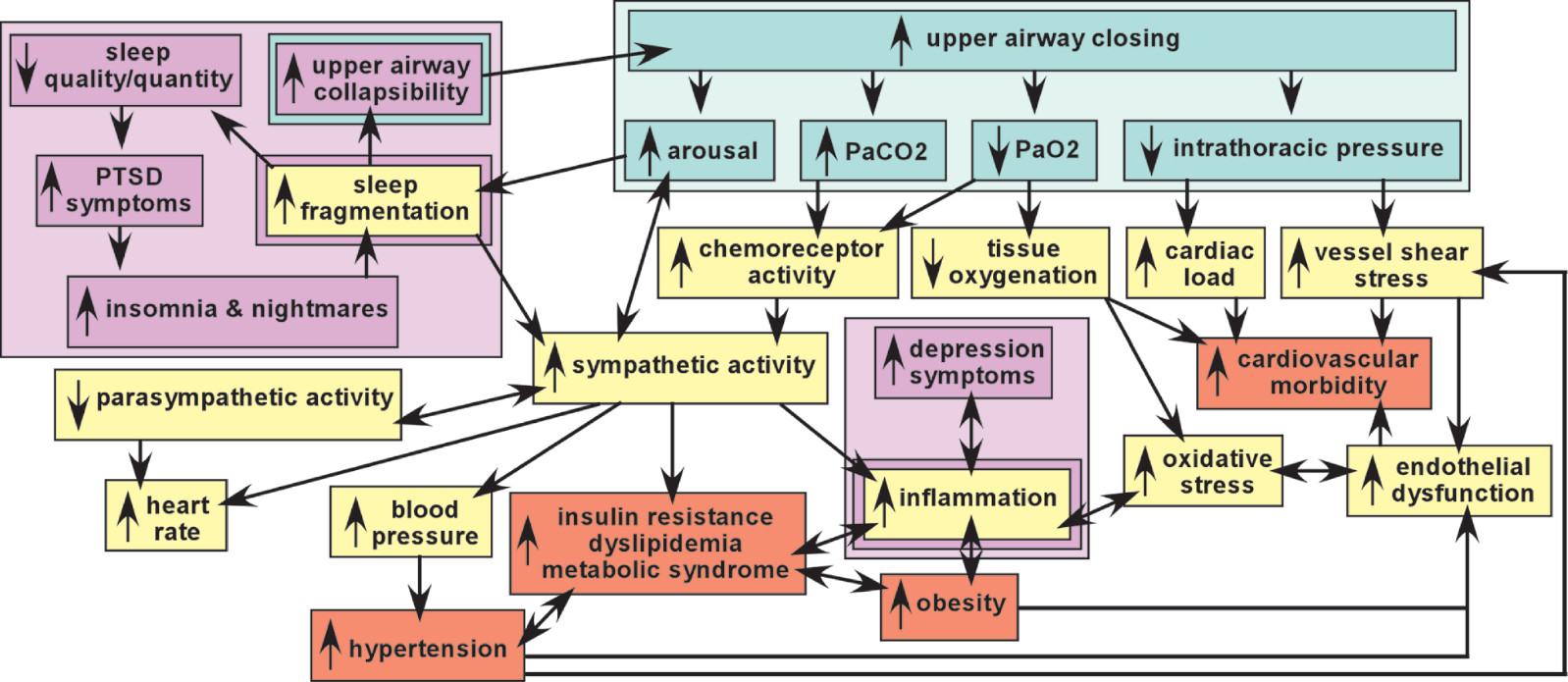
6,23Broadly defined, the sleep apneas are conditions in which breathing is repeatedly interrupted during sleep. OSA is defined by intermittent upper airway closings that cause partial reductions (hypopneas) and complete cessations (apneas) in breathing of ten or more seconds.
2,10,26,27 The most common metric for severity of OSA is the average number of apneas and hypopneas per hour of sleep (apnea-hypopnea index, AHI), with an AHI ≥5 required for diagnosis.
10 AHI of 5–15 is mild OSA, 15–30 is moderate OSA, >30 is severe OSA. These episodes are associated with a rapid decrease in blood oxygen level (hypoxia) and increase in blood carbon dioxide level (hypercapnia), which trigger (via chemoreceptor activation) an increase in arousal (respiratory effort-related arousals) and activation of the sympathetic nervous system that disrupts sleep and restores breathing (
Figure 1). The resultant sleep fragmentation may produce excessive daytime sleepiness. As noted above, OSA accompanied by daytime sleepiness is called OSA syndrome (OSAS).
10 Although the cycles of intermittent hypoxia have generally been thought to be the major source of injury, this has been questioned.
28–32 In brief, intermittent exposure to mild hypoxia has been utilized in both animal and human studies to induce adaptive responses (preconditioning) that increase resistance to injury by subsequent more severe exposures. Other aspects of OSA that could contribute to tissue injury include effects of sleep disruption itself, sympathetic over-activation, and increased cardiac afterload secondary to changes in intrathoracic pressure (
Figure 1).
1–3 Continuous positive airway pressure (CPAP) therapy during sleep prevents the episodes of airway collapse by delivering constant mild air pressure. With adequate compliance, CPAP is an effective treatment for OSA.
Epidemiology
OSA was once thought to be a relatively rare sleep disorder, with prevalence estimates ranging from 0.7%−3.3%. However, as noted in a recent review of epidemiological studies, the low prevalence reported in earlier studies resulted from the flawed assumption that OSA occurred only among individuals sufficiently symptomatic (e.g., snoring, daytime sleepiness) to be referred for evaluation.
10 Excessive daytime sleepiness along with other factors such as snoring and being male were previously considered “classic” signs of the disorder. These factors were utilized in polysomnography studies to prescreen for possible OSA, excluding any without the classic symptoms.
10 This led to an underestimation of the prevalence of the disorder, as the field now accepts that OSA can occur without any of these signs. More recent epidemiological studies have captured both the presence of OSA (AHI ≥5) and OSAS (OSA plus daytime sleepiness).
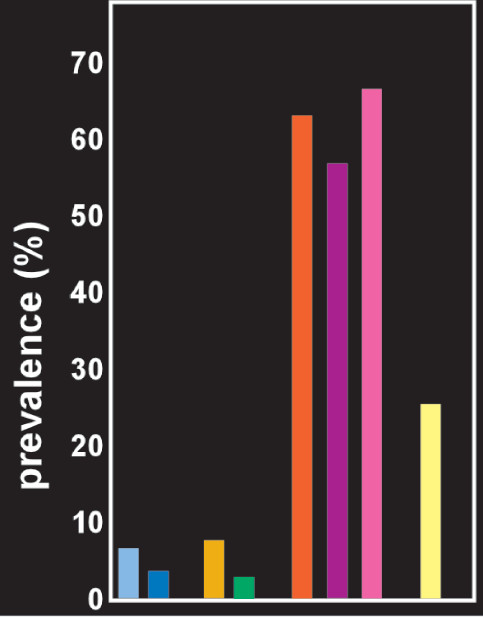
10 Based on eleven population-based epidemiological studies, the mean prevalence of OSA (i.e., AHI ≥5) was 22% in men and 17% in women. The mean prevalence of OSA syndrome (i.e., AHI ≥5 with excessive daytime sleepiness) was 6% in men (
Figure 2) and 4% in women.
10 Thus, approximately two thirds of individuals with OSA do not exhibit daytime sleepiness. There is considerable evidence of higher prevalence of OSA in patients with mental health disorders.
14 Studies in multiple settings have reported a much higher prevalence of OSA in patients with PTSD (
Figure 2).
9,11–13 Reported prevalence of OSA for other mental health conditions is also elevated, but varies with the setting and the disorder.
33–35 A systematic review reported that for studies conducted in clinic populations the pooled prevalence of OSA in patients with serious mental illness (major depression, bipolar disorder or schizophrenia) was 25.7%.
14 Prevalence was highest for major depression (35.2%) and lowest for schizophrenia (15.4%). Several hypotheses have been proposed for causal mechanisms linking OSA and psychiatric and somatic disorders (
Figure 1).
4–8 It is important to note that the defining criteria for a hypopnea episode developed by the American Academy of Sleep Medicine have evolved over time, resulting in clinically significant differences in AHI.
36,37 As discussed in both these studies, the older definitions underestimate the AHI compared with the newest, indicating the importance of close attention to the method of determining AHI when comparing clinical research studies from different eras.
36,37Studies Comparing Patients With OSA to Healthy Individuals
The vast majority of research studies have utilized a cross-sectional design in which groups of treatment naïve patients with OSA are compared with groups of healthy individuals. It is important to note that studies have utilized a wide range of criteria (inclusion, exclusion, matching) to select participants, resulting in comparison groups (OSA, healthy) that differed on potentially important variables (e.g., body mass index [BMI], sleep disturbance, medical conditions, psychiatric conditions, age, gender, education).
15,38 In addition, tests of effort were not incorporated into neuropsychological assessment batteries. This contravenes two of the main advisory and credentialing agencies for clinical neuropsychology, which hold the position that validity testing is a necessary aspect of any neuropsychological examination.
39,40Many studies have sought to determine whether OSA is associated with specific cognitive deficits using neuropsychological assessment. Meta-analyses and reviews have reported cognitive impairments related to OSA in multiple domains (e.g., attention, executive functioning, episodic memory, working memory, and processing speed).
16,41–43 For example, a recent meta-analysis of nineteen studies found that patients with OSA performed significantly poorer than healthy controls on all cognitive domains assessed except perception (e.g., Digit Cancellation Task), with the most affected domains being nonverbal memory (e.g., Rey Complex Figure recall), concept formation (e.g., WAIS-R Picture Arrangement), and psychomotor speed (e.g., Symbol Digit Modalities Test).
43 However, no consistent cognitive profile seems to have emerged.
21,44–48 Such studies are important for identifying and quantifying the types of cognitive deficits that may be evident in patients with OSA. They do not illuminate the aspects of OSA causing cognitive impairment. It is well established that other sleep disorders, common mental health disorders such as anxiety, and common substances of abuse such as alcohol can also negatively impact cognitive functioning.
49–51 For example, researchers generally cite intermittent hypoxia as a major mechanism of cognitive dysfunction and brain injury in OSA.
52 However, a review of thirty-seven articles concluded that sleep fragmentation was a stronger predictor of attention/vigilance deficits than hypoxemia.
53 Similarly, a meta-analysis of twenty-five studies found no relationship between severity of OSA (defined by the number of apneic and/or hypopneic events per hour of sleep) and cognitive deficits.
44 Relatedly, one study found that treatment-naïve OSA patients with higher levels of hypoxemia performed similarly or better on cognitive assessments than did carefully matched treatment-naïve OSA patients with lower levels of hypoxemia.
54 Knowing the specific pathophysiology of any cognitive impairment in OSA may be important for understanding the expected course and prognosis of such impairment, as well as treatment recommendations. Sleep disruption alone (e.g., repeated sleep fragmentation or complete disruption/cessation of sleep) is associated with altered functioning, ranging from cognitive impairments (e.g., sustained attention or vigilance) to affective disturbances and impairments in gene expression and metabolism.
55 A study in adults older than 60 years of age found that sleep efficiency was significantly related to decreased performance on the N-back task, whereas higher AHI and lower arterial oxygen saturation were not.
56Two longitudinal studies examining relationships between baseline measures of sleep and changes in cognition over time have reported conflicting results.
57,58 In one study, women over 65 years of age were screened for OSA and administered cognitive tests, then reassessed a mean of five years later.
57 OSA was associated with higher risk (odds ratio 1.8) of developing mild cognitive impairment or a full neurocognitive disorder. Although the groups did not differ on important medical comorbidities (e.g., history of heart disease or stroke, BMI, etc.), women who developed a cognitive problem had lower baseline cognitive scores than women who did not. Measures of sleep disruption (e.g., sleep fragmentation, arousal index, total sleep time) were not related to cognitive impairment. Two measures of hypoxia (higher oxygen desaturation index, higher percent sleep time in apnea or hypopnea) were related to cognitive impairment, one (sleep time with oxygen saturation below 90%) was not.
57 In the other study, a middle aged (45–64 years) population based cohort was followed for a median of 14.9 years.
58 Neither OSA severity nor any sleep index (measures of hypoxemia, disordered breathing, sleep fragmentation, sleep duration) were associated with either cognitive decline or any cognitive test.
A wide variety of structural and functional neuroimaging methods have been utilized in cross-sectional research studies seeking to identify OSA-related changes in the brain. Although most studies have concluded that OSA has detrimental effects on multiple brain regions, there has been relatively little anatomic consistency across studies.
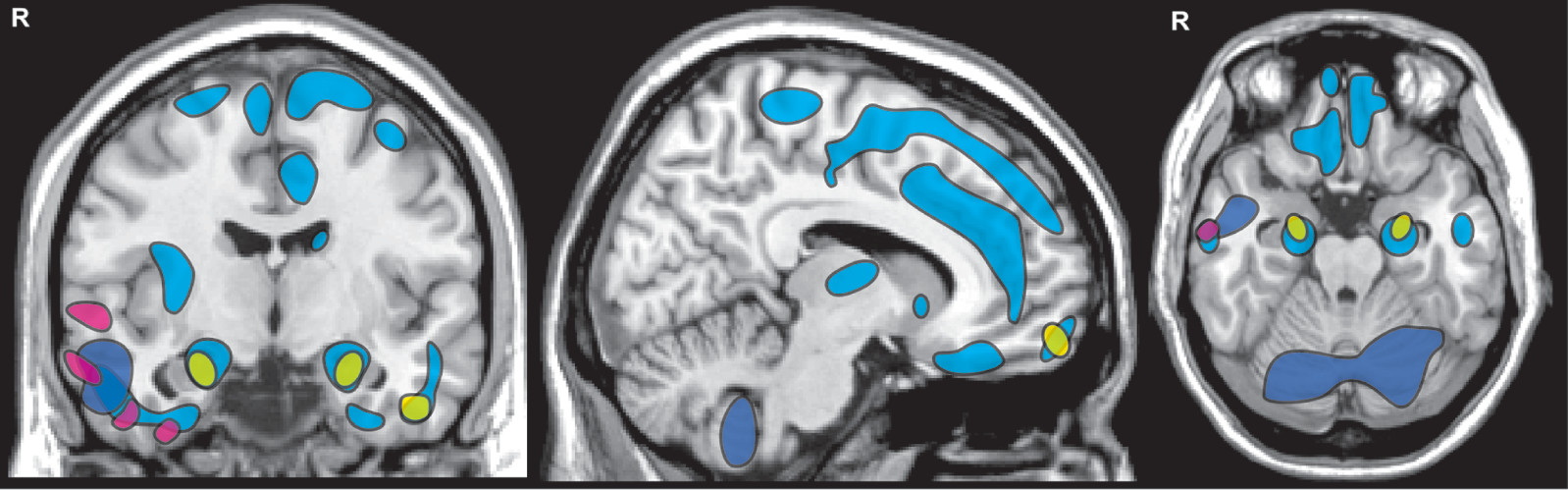
15,16 A meta-analysis (activation likelihood estimation, ALE) of magnetic resonance imaging (MRI) studies that utilized voxel based morphometry (VBM) found significant volume reductions in only four areas: bilateral hippocampal region, right frontal pole, left middle temporal gyrus (
Figure 3).
17 A later methodological review concluded that only three of the studies included in the meta-analysis met predetermined criteria for robustness.
18 As was noted in the review, there was little consistency in findings among the three (
Figure 3).
19–21 A more recent meta-analysis utilized ALE to identify anatomic areas implicated by both structural (VBM) and functional (fMRI) studies as altered in OSA.
59 The few areas of convergence (ventral insula, amygdala/hippocampal region) were only found for the right hemisphere. A study combining structural (VBM) and functional (arterial spin labeling [ASL]) MRI methods in which the comparison groups (mild OSA, moderate-severe OSA, healthy controls) were well matched on both BMI and sleep (e.g., daytime sleepiness, sleep quality) did not find any significant differences in either global or regional VBM measures.
60 Although global perfusion measures did not differ, there were several areas of significantly decreased gray matter perfusion in the moderate-severe OSA group compared with the control group. Similarly, a study that used single photon emission tomography (SPECT) to measure cerebral blood flow reported significant differences between the severe OSA and control groups, but not between the control group and either the mild or moderate OSA groups.
26 However, the areas identified in these two studies were quite different.
Longitudinal Studies of Treatment-Related Changes
From the clinical perspective, a key question is whether the various differences in brain function and structure are reversible or permanent. This is best addressed by longitudinal studies that compare measures prior to and following CPAP treatment (within-subjects design) in OSA patients with high adherence.
15 As would be expected, improvements in cognitive ability following CPAP treatment have been found to be related to level of CPAP use. For example, memory-impaired OSA patients using CPAP >6 hours per night on average were 7.8 times more likely to experience a normalization of memory abilities than memory-impaired OSA patients using CPAP <2 hours per night on average.
61 Multiple studies have reported treatment-related normalization of neuropsychological functioning and/or neuroimaging metrics in OSA patients with good CPAP utilization, although there has not been strong consensus regarding which domains of cognitive functioning improve.
46–48,62–64 A study that combined electrophysiological (EEG) and neuropsychological assessments of treatment naïve patients with severe OSA reported decreased power in the delta band associated with reductions in daytime sleepiness following three months of CPAP treatment.
62 Simple attention, processing speed, immediate verbal and delayed visual memory were also improved. A randomized pilot study of elderly patients newly diagnosed with severe OSA reported that the group receiving three months of CPAP treatment improved on multiple domains of neuropsychological functioning, whereas the group receiving conservative care did not.
63 Although there were no baseline differences in cortical thickness between the OSA groups, several regions were thinner in the conservative care group at three months. As noted by the authors, these findings indicate treatment-associated benefits to both cognitive functioning and brain structure.
63 A study utilizing deformation based morphometry to quantify brain volumetric changes associated with CPAP treatment in patients with moderate to severe OSA reported volume increases in multiple regions.
64 For several areas, longer periods of treatment (mean 18.2 months, range 8–44) were associated with greater increases in volume. A set of studies that obtained both functional and neuroimaging measures in patients with severe OSA after three and twelve months of CPAP treatment is particularly informative.
46,48 Although these studies reported partial or full normalization (compared with healthy controls) in both types of metrics, the temporal patterns of change differed. The greatest improvements on functional measures (neuropsychological tests of memory, attention, executive function; sleep; depression; quality of life) and in gray matter volume occurred during the initial three months of treatment. White matter integrity improved more between three and twelve months of treatment. As noted by the authors, the observed temporal differences in treatment-related recovery indicate the importance of utilizing multiple outcome measures when assessing efficacy.
48There is considerable evidence that effective treatment of a sleep disorder can improve symptoms of comorbid psychiatric and somatic conditions. A systematic review and a meta-analysis of clinical trials utilizing cognitive behavioral therapy for insomnia (CBT-I) concluded that it had beneficial effects on both sleep and psychiatric symptoms.
65,66 The systematic review included sixteen studies that evaluated CBT-I in patients with insomnia comorbid with a variety of psychiatric condition (e.g., depression, anxiety, PTSD, alcohol dependence).
65 The meta-analysis also reported that CBT-I was more effective at improving symptoms of comorbid psychiatric conditions than comorbid medical conditions (e.g., cancer, fibromyalgia, chronic disease).
66 Improved sleep following CBT-I was also related to decreased suicidal ideation and depressed mood in another study.
67 It is not uncommon for OSA and insomnia to be comorbid. A recent study in active duty military personnel utilizing both CBT-I and CPAP reported that improved sleep was associated with decreases in symptoms of depression and post traumatic arousal.
68 In addition, improved sleep has been associated with beneficial changes in trophic and inflammatory blood biomarkers in several studies.
68–70