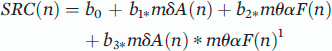Drowsiness has been defined as the progressive loss of cortical processing efficiency that occurs with time passing while awake.
1 The onset and progression of drowsiness is associated with a broad range of cognitive disturbances: slowed reaction times, reduced vigilance, and deficits in information processing including difficulties in integrating information and decreased accuracy of short-term memory.
2–4 Behavioral changes in attention and short-term memory are accompanied by alterations in brain activation as detected on functional magnetic resonance imaging.
5–7 A given cognitive function may decline, then return to baseline, then later decline again; the overall pattern remains one of widespread and progressive dysfunction. The precise mechanism(s) by which these cognitive declines take place has not been fully elucidated. However, part of the answer may lie in the realization that sleep is a much more intricate process than previously believed. Sleep-like states occur within cortical columns, and the likelihood that a given column will enter such a state is related to prior activity.
8–10 Local sleep states can arise in larger areas or regions of cortex, or even involve one hemisphere at a time.
11,12 The appearance is patchwork; localized wake-like states can be observed during whole-animal sleep
8 and localized sleep-like states occur at times during whole-animal waking.
13,14 The accumulation of sleep-regulatory substances, produced as a consequence of the normal metabolic activity of neural networks, appears to trigger the entry of cortical columns into local sleep.
15 In the rat, this is reflected in islands of increased delta power on EEG appearing during wakefulness, concurrent with sharp drop-offs in multiunit activity.
16Studies examining drowsiness and sleep have generally focused on spectral power within a given frequency band rather than on correlations related to a specific frequency. In humans subjected to prolonged wakefulness, changes to scalp EEG in the form of increased theta power (5–7 Hz) have been seen, as well as increased spectral power in the delta band (0.5–4 Hz) during subsequent sleep.
17,18 Scalp EEG analysis of the full sleep-wake cycle has demonstrated a negative correlation between theta power and self-rated alertness.
18 The posterior dominant alpha frequency changes its morphology, spatial distribution, and frequency with varying levels of alertness.
19 Clinical observation reveals a pattern of slowing of the alpha frequency with the onset of drowsiness, accompanied by an increase in amplitude.
20 While much of the published work to date has focused on alpha oscillations having a negative correlation with local cortical excitability, the alpha phase and frequency appear to have a role in synchronization of cortical regions, particularly those involved in attention and executive processing.
21,22 Regarding the assessment of local sleep while awake in humans, high-density scalp EEG recordings have shown localized experience-dependent increases in theta power regionally at roughly the lobar level.
23 Studies using stereoelectroencephalography have revealed evidence of arousal-like activity in the hippocampus during non-REM sleep, the detection of hippocampal sleep spindles prior to their neocortical appearance, and islands of wake-like activity in primary motor cortex during sleep,
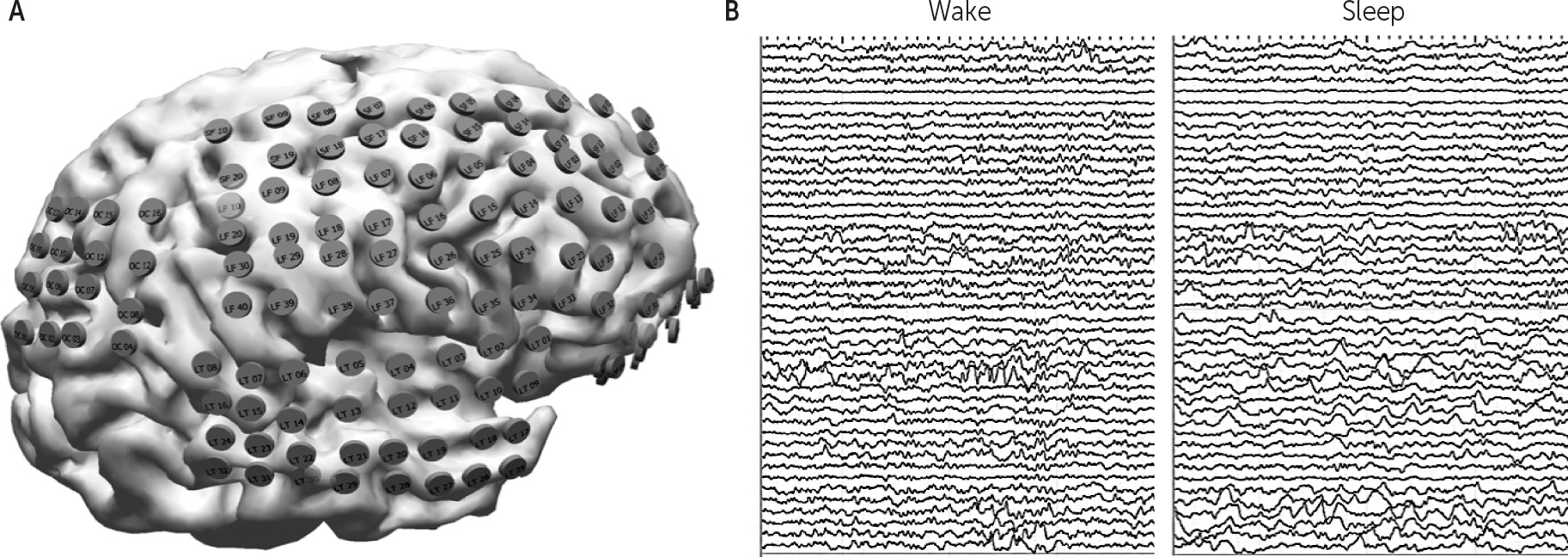
24–26 but to date, human evidence of local sleep while awake at the level of the local field potential using electrocorticography (ECoG) has not been demonstrated.
Given the visible slowing of the scalp-recorded alpha frequency with drowsiness,
20 the postulated role of alpha frequency in governing attention and the hypothesis that drowsiness is the clinical manifestation of progressively increasing local sleep while awake, we hypothesized that combining features of focal regions with increased delta power and decreased alpha frequency might create a way to define areas of relative localized inactivity, as a surrogate marker for local sleep, during the wake state. More simply, the combination of the power within one frequency band and the specific frequency within another band might serve as a better descriptor of the cortical state than either alone. Given that the classic “Berger” bands are average ranges derived from decades of clinical experience, and using alpha as an example, the actual frequency in question commonly slows to less than 8 Hz during periods of drowsiness, we additionally examined (along with the classically defined separate beta, alpha, theta and delta bands) three combination bands of beta/alpha, alpha/theta and theta/delta. We further hypothesized that applying the Hilbert-Huang transform
27 to the bandpass filtered signals (in order to derive both instantaneous amplitude and frequency) would enhance temporal resolution. We tested all band combinations of amplitude and frequency with a clustering algorithm comparing a period of clear sleep to one of clear wakefulness for the purpose of identifying the combination of the ECoG signals that provided the greatest separation between the two states, with the goal of creating a reliable signature of the active versus inactive state locally for each electrode.
Discussion
The functional deficits that appear with prolonged wakefulness fluctuate in severity over time,
6 and the effect on cognitive function is not uniform with respect to the type of function.
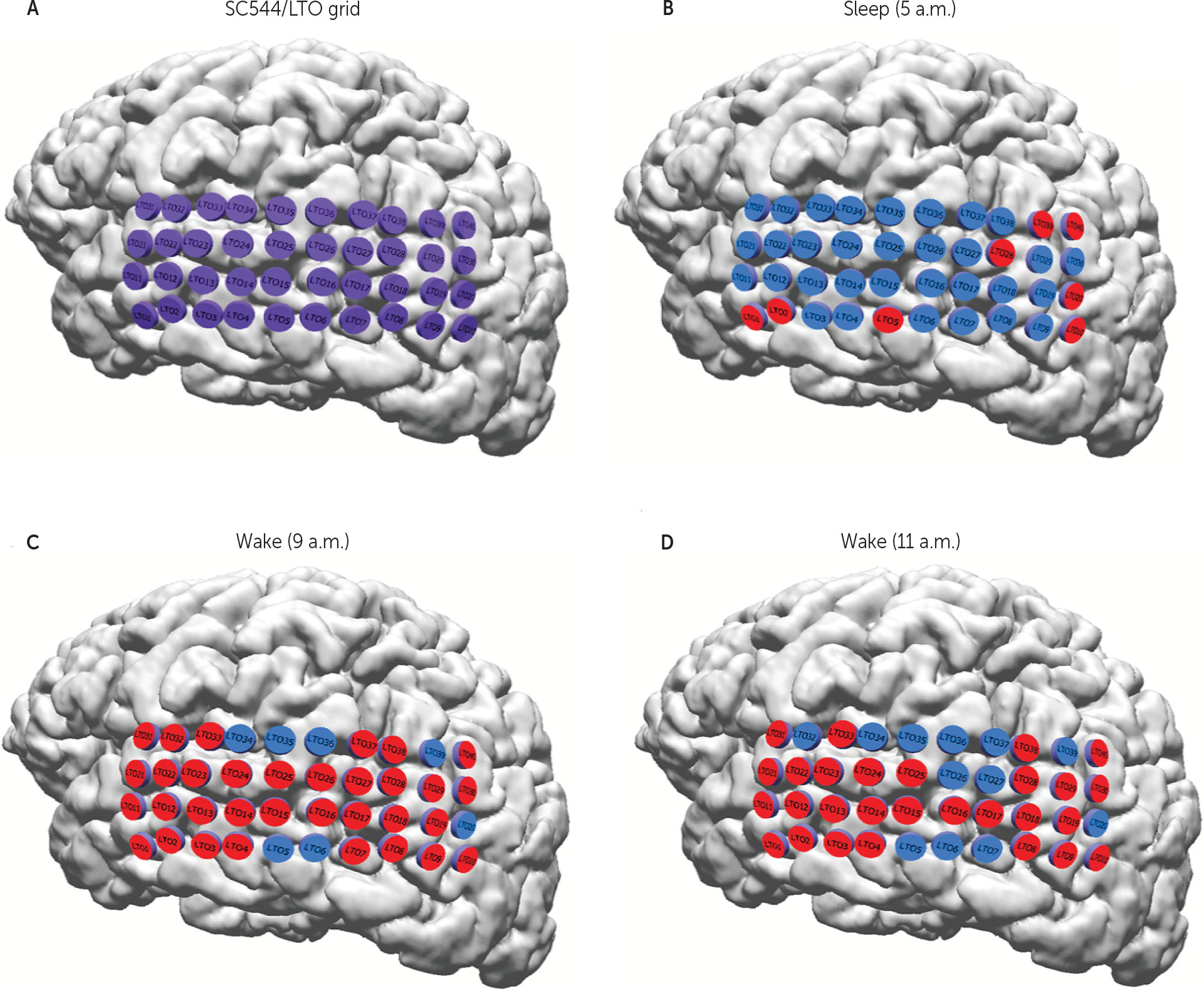
31 Isolated areas of cortex episodically entering an inactive state during the overall awake state of the brain provide an explanation for this variability. To our knowledge, this is the first ECoG study in humans demonstrating evidence of local sleep-like changes during the wake state. Consistent with what has been demonstrated in animal models, we show a dynamic pattern of transitory focal resting states at the level of the local field potential.
It is widely accepted that a robust signature of the sleep state is the increase in
δ band spectral power.
17,18 In this study, we propose a broader set of signatures to track changes to ECoG with time awake (as a surrogate marker for drowsiness): the instantaneous amplitude in
δ band and the instantaneous frequency in
θα band. These features were selected after a thorough statistical analysis of the change in
IA and
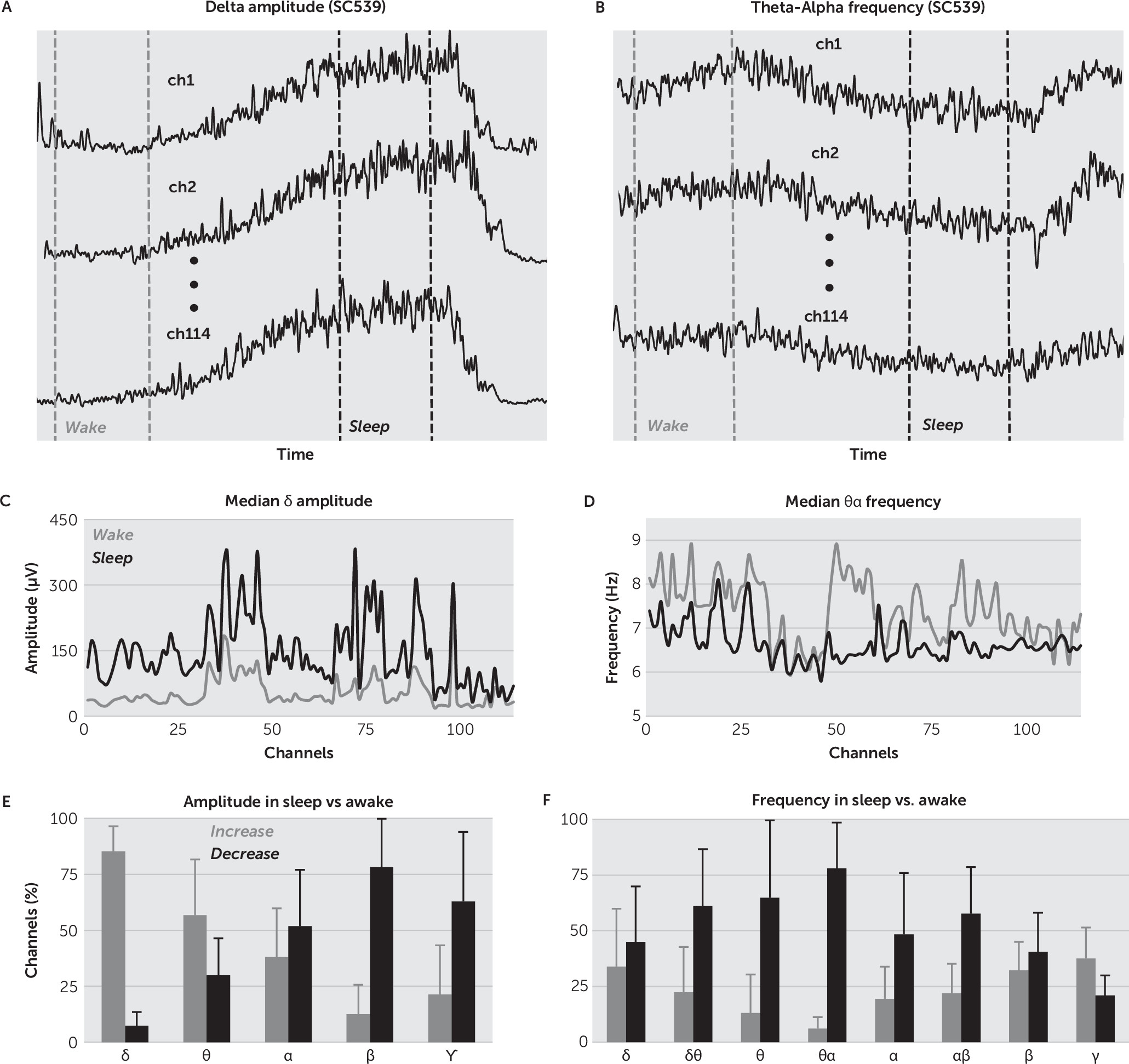
IF in all the EEG bands. We found that the maximum percentage of channel increase in amplitude in sleep versus awake was in the
δ band, and the maximum percentage of channel decrease in frequency was in the
θα band (
Figure 2E–
2F).
We used the normalized, smoothed
δ band
IA,
δA, together with the smoothed
IF in
θα band,
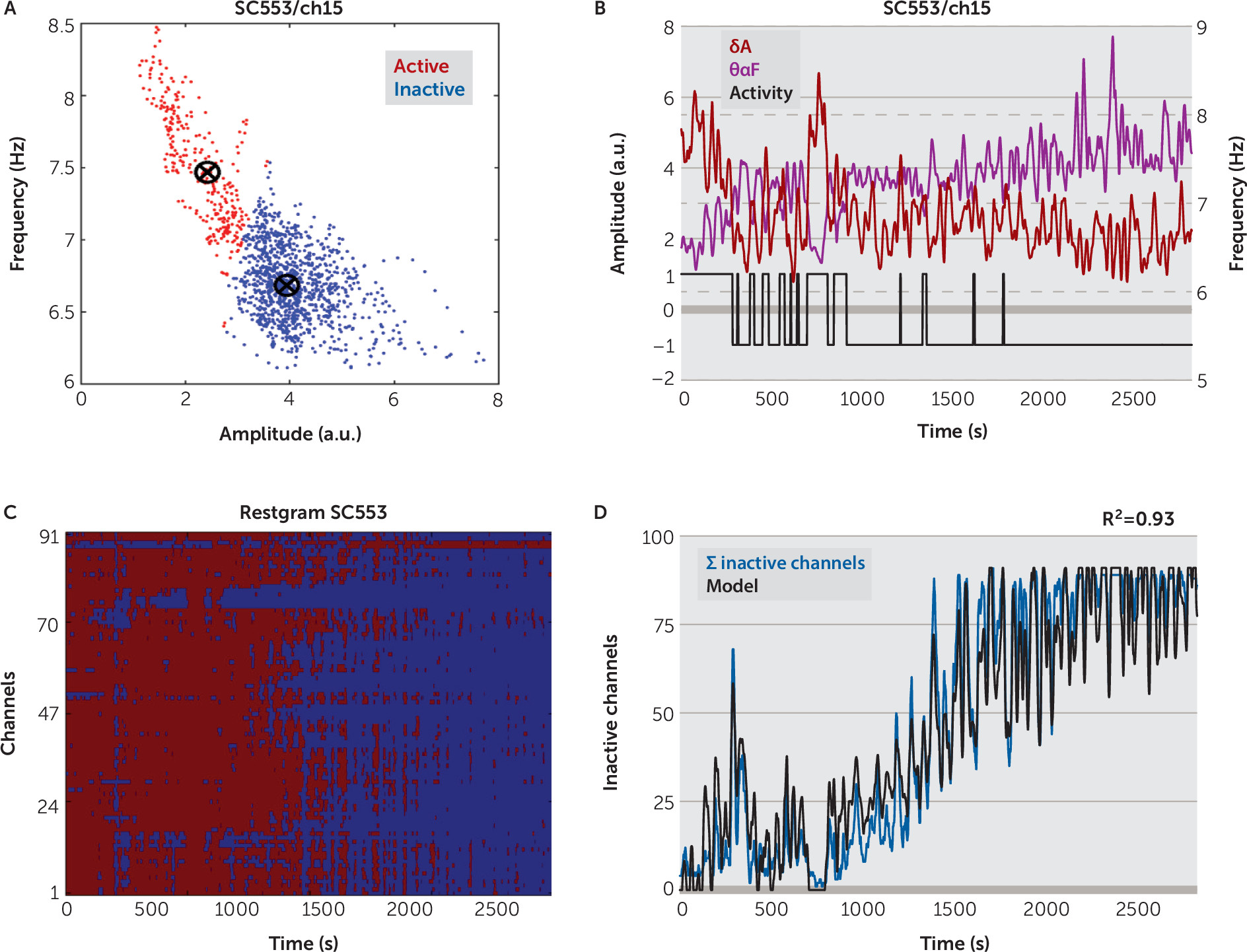
θαF, to classify the state of each channel as being active or inactive (
Figure 3A–
3B). We assembled the results of all channels’ state classification in a matrix form, called
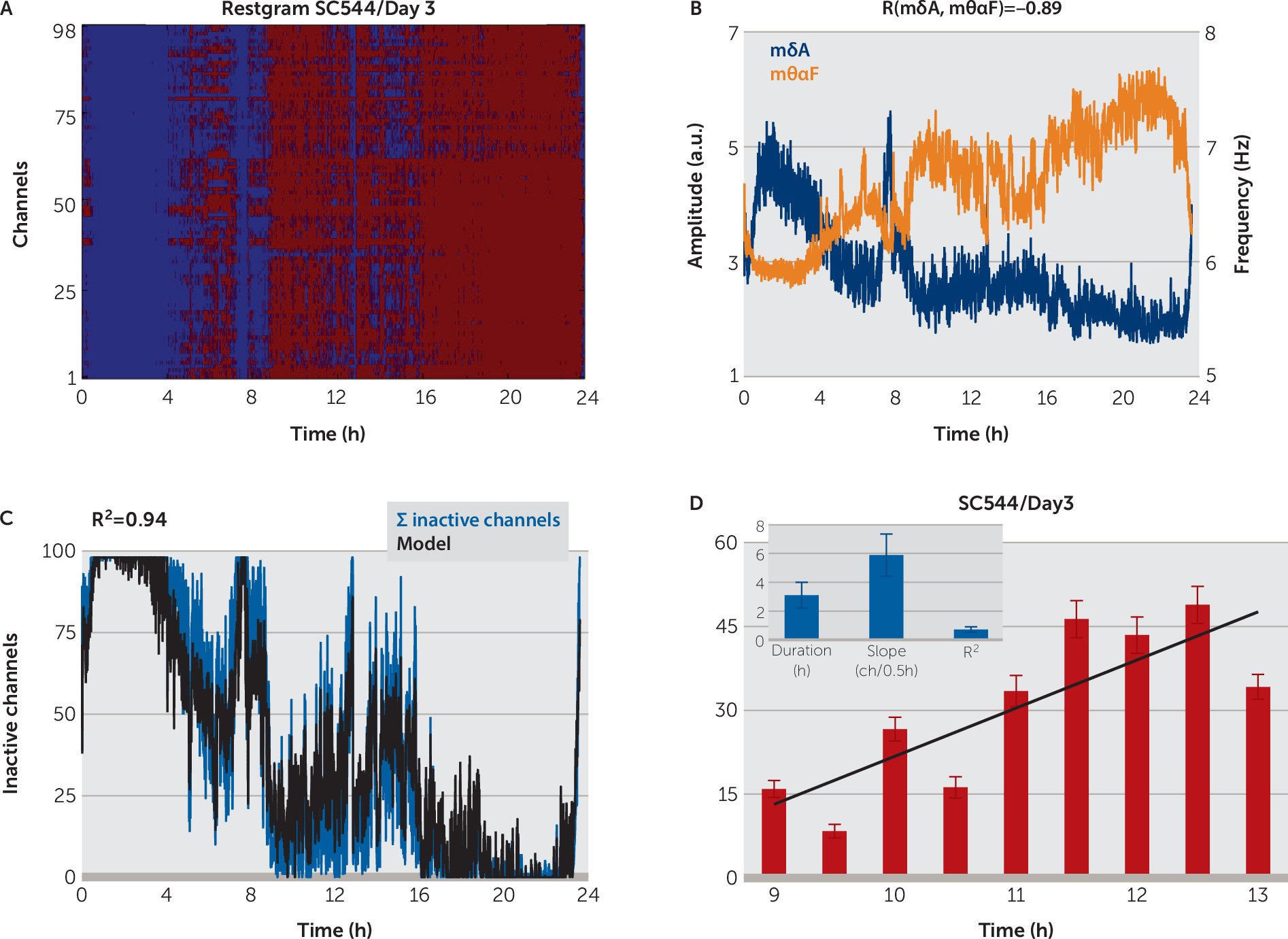
restgram, which shows evidence of local sleep: during the wake intervals, established independently by analyzing the video records, the number of inactive channels was significantly smaller than during the sleep intervals, while during sleep most of the channels were inactive (
Figure 3C,
Figure 4A, also see Figure S4B in the
online data supplement).
Sleep-state classification at the channel level allowed us to define a novel measure of local sleep: the sum (count) of the inactive channel,
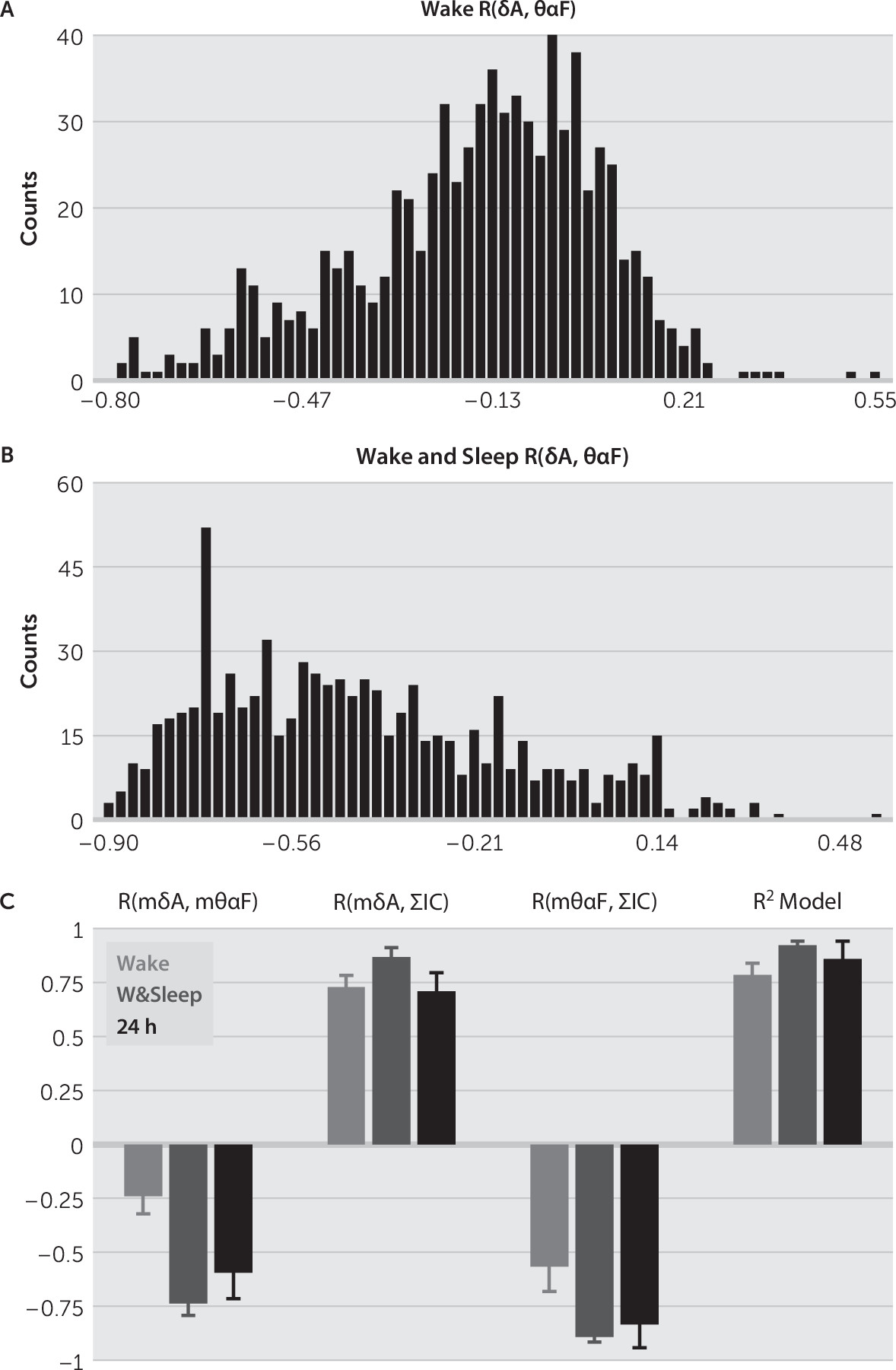
ΣIC, computed for every 2 seconds (or 10 seconds for 24-hour records). Correlation analysis revealed that
ΣIC, the average across channels of normalized, smoothed
IA in
δ band (
mδA), and the average across channels of smoothed
IF in
θα band (
mθαF), were pairwise strongly correlated (
Figure 6C). Due to these correlations, we were able to approximate
ΣIC with high accuracy by using only the global variables
mδA and
mθαF (
Figure 3D,
Figure 5, [also see Figure S4C–S4D in the
online data supplement). After the model parameters were correctly determined in a session that includes a wake and a sleep interval, one could estimate the percentage of the cortex in a sleep state, at any given time, by using only two global variables,
mδA and
mθαF. Therefore, we have established a reliable metric corresponding to the percentage of channels in an inactive state. Whether this metric has a direct correspondence to the progressive performance degradations associated with drowsiness is the subject of ongoing study, but it cannot be concluded from the current work.
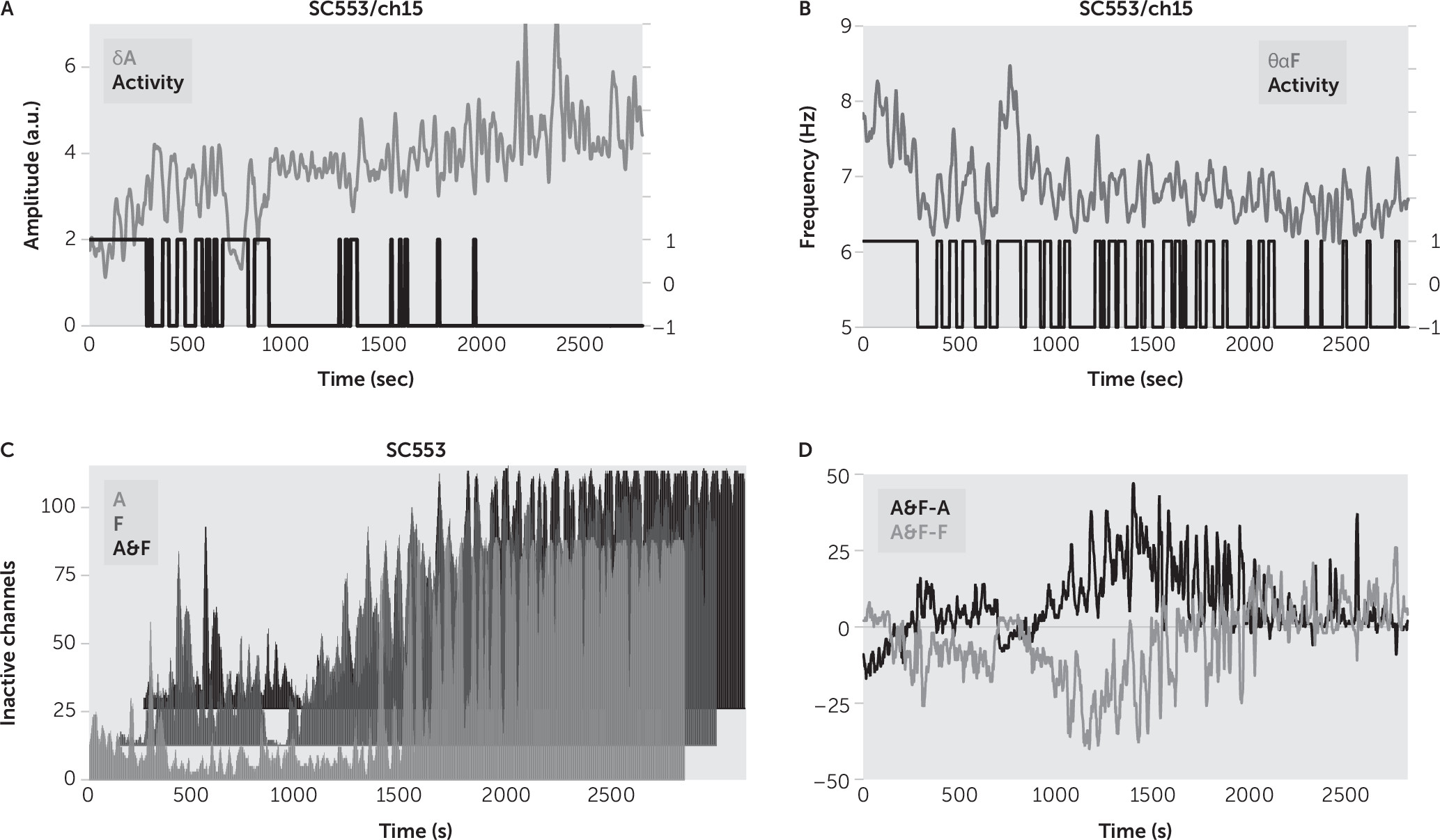
As an inspection, the analysis was rerun with a single feature like
δA (
Figure 4A), or
θαF (
Figure 4B), followed by one-dimensional
k-means clustering, to determine the wake state of channels. There is no standard measure of local sleep, and thus we could not objectively establish whether it was better to use both features (
δA together with
θαF) than using only one of them for channel sleep detection. We reasoned that because
δA and
θαF were strongly correlated during sleep (
Figure 6B), we would have a more robust classification when using both features (e.g., for the sleep state, we should have an increase in
δA simultaneously with a decrease in
θαF), than when using only one of them. We found that the sum of inactive channels was overestimated for amplitude sleep detection and underestimated for frequency sleep detection, in comparison with the proposed amplitude-frequency sleep detection (
Figure 4C–
4D). Thus, we concluded that the proposed sleep detection gave less noisy results in comparison with sleep detection based on one feature.
A significant difference between this work and prior studies is the combination of instantaneous frequency and instantaneous amplitude. The two band combinations were analyzed specifically to allow tracking of the frequency in those instances where it might drift above or below the “official” limits of the Berger bane. Even in Hans Berger’s original description of the alpha frequency, the bandwidth he wrote of was 7.812–13.28 Hz, with a lower limit than is currently used.
32 Our results are consistent with the idea that the frequency in the theta to alpha range has a direct relationship to the occurrence of local sleep—the high predictive value of a linear regression model for the number of electrodes in the sleep state suggests that generation of this frequency is directly dependent on the percentage of cortex in the wake state at any given time. Given the evidence for alpha phase dynamics modulating neuronal stimulus-response, stimulus-perception, and speed of processing (see
22 for a review), the slowing of the alpha frequency into the theta range occurring with increasing drowsiness may provide an additional explanation for the development of functional deficits, particularly with respect to attention and executive function.
Limitations of the current study include the fact that it is a retrospective analysis. As such, formal behavioral or repetitive performance testing was not available to provide a clinical correlate to the measured percentage of local sleep. As sleep staging is not routinely performed in patients undergoing invasive monitoring prior to surgery, no consistent placement of scalp and electromyogram electrodes was made, preventing formal sleep staging. A weakness shared with almost every study involving human ECoG is that the patient population studied has medically refractory epilepsy. Given that refractory epilepsy is the only condition for which this type of long-term invasive monitoring is appropriate, this limitation is inescapable.
To address the limitations of the present study, a prospective study with formal sleep staging and repetitive behavioral analysis and performance testing is planned for the future. Greater numbers of subjects may allow for meaningful regional analysis of the spatial distribution of the inactive states.
The present study provides additional support for the concept of local sleep while awake, the first based on intracranial subdural grid recordings in humans. Rather than simply a global process imposed on the brain, sleep develops as a local process within the cortical column due to the accumulation of sleep regulatory substances.
33 These appear as the consequence of normal neuronal network activity, and the greater the activity, the greater the likelihood of entering the sleep state.
34 The present results are consistent with the clinically observed gradual decline in cognitive abilities that is irregular with respect to both spatial and temporal progression, commonly described as drowsiness.