Preliminary Evidence on Cannabis Effectiveness and Tolerability for Adults With Tourette Syndrome
Abstract
Methods
Results
Clinical Characteristics and Cannabis Use
| ID | Age (Years)/Gender | Comorbidities | Current and Past Medications | How Participant Learned About Cannabis for Tics | Pharmaceutical Cannabinoid | Cannabis | |||
|---|---|---|---|---|---|---|---|---|---|
| Current Source | Dosing | Route | Duration of Single Dose Effect on Tics (Hours) | ||||||
| 1 | 19/M | OCD, ADHD, restless leg syndrome, nocturnal myoclonus | Past: clonidine, risperidone, sertraline, amphetamine/dextroamphetamine, atomoxetine, clonazepam, desmopressin | Mother found information online | None | Approved supplier | 0.5 g four times daily | Smoked | 2–3 |
| 2 | 40/F | OCD, depression | Current: citalopram, clonazepam; Past: clonidine, risperidone, pimozide, aripiprazole, quetiapine, tetrabenazine, paroxetine, fluoxetine | Family friend with TS | Past: nabiximols, nabilone | Approved supplier | 0.125–0.5 g in food every night; 0.5 g smoked/day | Smoked, ingested with food | 6 |
| 3 | 21/M | OCD, anxiety | Past: olanzapine, quetiapine, methylphenidate, atomoxetine, fluoxetine, bupropion | Personal experience | None | Unapproved supplier | 1 g three times daily | Vaporized, ingested with food | 3–4 |
| 4 | 32/M | ADHD, ASD, hypertension | Past: clonidine, risperidone, haloperidol, pimozide, fluoxetine | TS clinic | Past: nabiximols | Approved supplier | 0.5 g every night for 1 week, then discontinued for 3 weeks | Vaporized | 24 |
| 5 | 34/M | OCD, social phobia | Current: venlafaxine Past: clonidine, pimozide | Friend | Past: dronabinol | Compassion clinic | 0.25 g daily | Vaporized | Uncertain |
| 6 | 51/M | OCD | Current: clonazepam Past: clonidine, mirtazapine, amytriptyline, lorazepam, valerian | Read research by Muller-Vahl and colleagues online | none | compassion clinic | 3 g in food, twice weekly | ingested with food | 5 |
| 7 | 28/M | OCD, ADHD, platelet abnormality | Past: risperidone | Personal experience | None | Unapproved supplier | <3 g spread over entire day | Smoked | Uncertain |
| 8 | 27/M | HIV positive | Current: elvitegravir/cobicistat/emtricitabine/tenofovir, guanfacine, clonazepam Past: clonidine | Personal experience | None | Compassion clinic | 0.1 g three times daily | Smoked | 2–3 |
| 9 | 18/M | OCD, ADHD, panic disorder | Current: sertraline Past: clonidine, methlyphenidate | Online | None | Unapproved supplier | 0.3 g spread over entire day | Smoked | 1.5–2 |
| 10 | 22/M | ADHD | Past: clonidine, risperidone, pimozide, quetiapine, methylphenidate, atomoxetine, bupropion, fluoxetine, clonazepam | Online | Past: nabilone | Unapproved supplier | 1.5 g spread over entire day | Smoked, ingested with food | 2 |
| 11 | 27/M | OCD, ADHD | Past: clonidine, methlyphenidate, fluoxetine, clobazam, prindolol | Personal experience | None | Approved supplier | 1.5–2 g 4–5×/daily | Smoked, ingested with food | 1–2 |
| 12 | 64/M | OCD, ADHD, COPD, type 2 diabetes | Current: citalopram, acetylsalicylic acid, clopidogrel, metformin, bisoprolol, amlodipine, atorvastatin, hydrochlorothiazide, omeprazole; Past: clonidine, amytriptyline, bupropion | Personal experience | Past: nabiximols | Approved supplier | 10 g/day | Smoked, ingested with food | 1–2 |
| 13 | 24/M | OCD, ADHD, depression | Past: clonidine, risperidone, haloperidol, methlyphenidate, atomoxetine, valproate, topiramate, fluoxetine, fluvoxamine, bupropion, zopiclone, clonazepam, lorazepam, diphenhydramine | Magazine | Past: nabiximols, nabilone | Unapproved supplier | 1 g/day smoked, 2 g/day vaporized | Smoked, vaporized | 2–3 |
| 14 | 30/F | Anxiety | Current: ziprasidone, clonazepam, sertraline, pimozide Past: risperidone, lurasidone, quetiapine, tetrabenazine, pramipexole, clomipramine, trazodone, zoplicone, lorazepam, melatonin, tetracycline, isotretinoin | Personal experience | None | Approved supplier | 0.2–0.4 g 1–2 times/week | Smoked | 2–4 |
| 15 | 24/M | ADHD, anxiety, depression | Current: fluoxetine Past: haloperidol, risperidone, olanzapine, quetiapine, clonidine, topiramate, fluoxetine, fluvoxamine, citalopram, methylphenidate, dextroamphetamine, atomoxetine, clonazepam, diazepam | Friend | Past: nabiximols, nabilone | Unapproved supplier | 0.6 g 4–5 times/day | Vaporized | 3 |
| 16 | 42/F | OCD, ADHD, PTSD, hepatitis C | Current: clonidine, aripiprazole, lisdexamfetamine, paroxetine, trazodone, clonazepam Past: ziprasodone, topiramate, bupropion | Personal experience | Past: nabilone | Approved supplier | 0.25 g 4–6 times/day | Smoked | 2 |
| 17 | 25/M | OCD | Current: haloperidol, fluvoxamine, acetaminophen/dextromethorphan/doxylamine Past: aripiprazole, lorazepam | Personal experience | None | Unapproved supplier | 1 g three times daily | Smoked | 4–6 |
| 18 | 32/M | OCD, ADHD, specific phobia (needle) | Past: clomipramine, imipramine, methylphenidate, sertraline, fluoxetine, paroxetine | TV: news report | None | Approved supplier | 1 g/day total: small amount ingested every few hours, vaporizer in PM | Vaporized, ingested with food | 3 |
| 19 | 49/M | none | Current: sildenafil Past: clonidine, pimozide, tetrabenazine, clonazepam, notriptyline, gabapentin, naproxen | TS clinic | Past: dronabinol, nabiximols, nabilone | Approved supplier | 0.5 g twice daily | Vaporized | 8–12 |
Effect of Cannabis on Tics and Related Symptoms
| ID | YGTSS Total Tic Score | YGTSS Impairment | Y-BOCS Total | ASRS Criteria for ADHD | CGI-S Score | CGI-I Score | Cannabis Impact on Comorbid Conditions | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | |||
| 1 | 26 | 0 | 40 | 0 | 17 | 0 | Yes | No | 5 | 1 | 1 | Decreased OCS, ADHD and rage |
| 2 | 46 | 20 | 50 | 40 | 27 | 16 | Yes | No | 7 | 4 | 2 | Decreased OCS and rage |
| 3 | 26 | 10 | 30 | 10 | 22 | 11 | Yes | No | 3 | 1 | 2 | Improved attention; decreased restlessness, impulsivity and rage |
| 4 | 44 | 18 | 50 | 0 | 12 | 12 | Yes | No | 4 | 2 | 1 | Improved attention and communication skills |
| 5 | 36 | 11 | 20 | 10 | 28 | 16 | Yes | Yes | 4 | 3 | 1 | Decreased OCD and anxiety; improved attention |
| 6 | 26 | 17 | 30 | 20 | 21 | 19 | Yes | No | 4 | 3 | 2 | Decreased OCD, anxiety, and rage; improved sleep |
| 7 | 21 | 8 | 20 | 0 | 0 | 0 | No | No | 4 | 2 | 2 | Reduced rage and irritability |
| 8 | 26 | 13 | 30 | 10 | 20 | 14 | Yes | No | 4 | 3 | 2 | Decreased ADHD and rage; improved sleep |
| 9 | 23 | 6 | 0 | 20 | 23 | 19 | No | No | 4 | 2 | 2 | Reduced rage |
| 10 | 30 | 18 | 30 | 10 | 0 | 0 | Yes | No | 5 | 3 | 2 | Reduced restlessness and anxiety; improved attention |
| 11 | 37 | 24 | 40 | 20 | 23 | 20 | No | No | 5 | 3 | 2 | Reduced OCD and ADHD; improved concentration and organization |
| 12 | 26 | 28 | 40 | 30 | 26 | 18 | No | No | 4 | 4 | 3 | Reduced OCD and restlessness; improved concentration and organization |
| 13 | 31 | 0 | 50 | 0 | 25 | 0 | Yes | No | 5 | 1 | 1 | Decreased OCD, ADHD and rage |
| 14 | 24 | 0 | 40 | 0 | 0 | 0 | Yes | No | 5 | 3 | 1 | Reduced rage |
| 15 | 35 | 0 | 50 | 0 | 35 | 0 | Yes | No | 6 | 1 | 1 | Decreased OCD, ADHD, and rage |
| 16 | 33 | 15 | 40 | 20 | 38 | 12 | Yes | No | 5 | 2 | 2 | Decreased symptoms of OCD and rage |
| 17 | 25 | 7 | 40 | 10 | 0 | 6 | No | No | 6 | 2 | 1 | Decreased anxiety and rage |
| 18 | 39 | 15 | 40 | 10 | 25 | 13 | Yes | No | 5 | 3 | 1 | Reduced anxiety and improved productivity |
| 19 | 26 | 21 | 30 | 10 | 14 | 3 | No | No | 4 | 3 | 1 | Decreased OCS |
| Instrument and Symptoms | Baseline (Mean±SD) | During Cannabis Treatment (Mean±SD) | Percentage Reduction From Baseline (%) | p | |
|---|---|---|---|---|---|
| YGTSS | Motor tic severity | 16.7±3.3 | 7.3±5.2 | 56.3 | <0.001 |
| Vocal tic severity | 13.8±4.4 | 4.8±4.4 | 65.2 | <0.001 | |
| Impairment | 35.3±12.6 | 11.6±11.2 | 67.1 | <0.001 | |
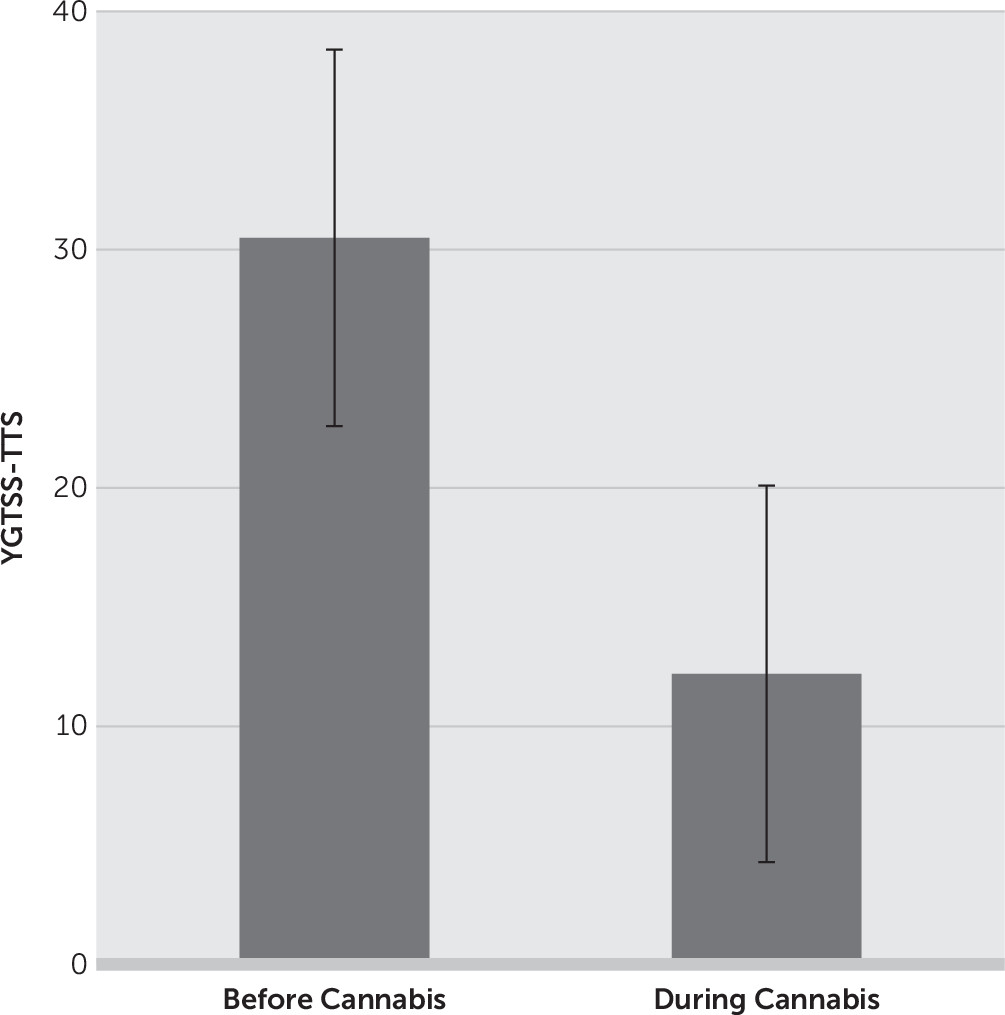
| Total tic severity | 30.5±7.2 | 12.2±8.6 | 60 | <0.001 | |
| Total severity scale | 65.8±17.7 | 23.7±18.1 | 64 | <0.001 | |
| Y-BOCS | Obsession subtotal | 9.5±6.0 | 5.0±4.5 | 47.4 | 0.001 |
| Compulsion subtotal | 9.2±5.8 | 4.5±3.7 | 51.1 | 0.003 | |
| Total | 18.7±11.7 | 9.4±7.8 | 49.7 | 0.001 | |
| PUTS | 26.2±4.9 | 18.6±4.6 | 29 | <0.001 | |
| Baseline (Number of Participants) | During Cannabis Treatment (Number of Participants) | Percentage Reduction From Baseline (%) | |||
| ASRS: Symptoms highly consistent with ADHD | 13 | 1 | 92.3 | <0.001 | |
| CGI-I: Much improved or very much improved | N/A | 18 | N/A | N/A | |
| CGI-S: Moderately to extremely ill | 18 | 2 | 88.9 | <0.001 | |
Cannabis Tolerability
| ID | Marijuana Effect Expectancy Questionnaire | Adverse Effects Based on Open-Ended Questions | |||||
|---|---|---|---|---|---|---|---|
| Cognitive and Behavioral Impairment | Relaxation and Tension Reduction | Social and Sexual Facilitation | Perceptual and Cognitive Enhancement | Global Negative Effects | Craving and Physical Effects | ||
| 1 | 2.6 | 2.3 | 2.3 | 2.3 | 1.0 | 2.5 | None reported |
| 2 | 3.3 | 3.1 | 3.0 | 3.3 | 2.7 | 4.2 | Increased sleepiness and reduced concentration |
| 3 | 2.4 | 2.4 | 1.7 | 2.1 | 1.3 | 3.0 | None reported |
| 4 | 3.2 | 3.3 | 3.0 | 3.3 | 2.8 | 3.3 | Increased irritability and appetite |
| 5 | 2.6 | 3.8 | 2.8 | 3.3 | 2.3 | 3.3 | Dry mouth and eyes |
| 6 | 2.4 | 3.3 | 2.4 | 2.4 | 1.4 | 2.7 | None reported |
| 7 | 1.7 | 3.1 | 2.0 | 2.8 | 1.1 | 2.5 | Less motivated, reduced short-term memory |
| 8 | 3.3 | 3.0 | 2.9 | 2.9 | 2.1 | 3.7 | Higher doses cause increased social anxiety and appetite |
| 9 | 3.3 | 2.9 | 1.9 | 2.8 | 1.3 | 3.0 | Decreased concentration and worsening of symptoms at higher doses |
| 10 | 3.8 | 3.9 | 2.2 | 2.8 | 1.4 | 3.0 | May impair attention, short-term memory, information processing |
| 11 | 1.9 | 2.9 | 1.9 | 1.8 | 1.1 | 2.3 | None reported |
| 12 | 2.7 | 3.1 | 2.1 | 2.9 | 1.7 | 3.2 | None reported |
| 13 | 1.7 | 1.4 | 1.9 | 2.0 | 1.3 | 2.0 | None reported |
| 14 | 3.5 | 3.4 | 2.2 | 3.4 | 1.4 | 3.5 | Increased anxiety if around others |
| 15 | 2.7 | 2.3 | 1.7 | 2.0 | 1.1 | 2.5 | None reported |
| 16 | 2.2 | 3.0 | 1.7 | 1.5 | 1.0 | 2.3 | None reported |
| 17 | 3.0 | 3.0 | 2.0 | 2.5 | 1.2 | 2.8 | Higher doses cause wheezing and the sensation of being “high” |
| 18 | 3.2 | 3.4 | 2.1 | 2.0 | 1.1 | 3.5 | Higher doses cause sleep problems, confusion, and “high” sensation |
| 19 | 2.6 | 2.6 | 2.3 | 2.4 | 1.3 | 2.8 | Feels “high” sometimes |
Discussion
Acknowledgments
Footnote
References
Information & Authors
Information
Published In
History
Keywords
Authors
Author Contributions
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

