Increasingly strong evidence supports longitudinal improvements in expression of symptoms in borderline personality disorder (BPD).
9,10 This is contrary to more dated clinical opinions that BPD is highly resistant to treatment and unlikely to remit. Multiple longitudinal studies indicate that BPD has a high rate of remission over time. In addition, many effective treatment approaches have been developed. Understanding the underlying factors that contribute to the development and persistence of the symptoms of BPD is critical to improving interventions and outcomes. In addition, elucidating possible biases and longstanding misconceptions may provide the foundation necessary to facilitate optimal treatment and provide directions for future research.
BPD is a severe psychiatric disorder characterized by instability of interpersonal relationships, self-image, and affect, as well as impulsivity.
11 It is associated with high risk behavior including impulsive and potentially self-harming actions such as unsafe sex, substance use, reckless driving, nonsuicidal self-injury (NSSI) and suicidal behavior. Over 70% of patients with BPD report a history of multiple episodes and methods of NSSI, while 60% report multiple suicide attempts.
12 Individuals with BPD tend to utilize mental health care services at a high rate.
13 BPD occurs in 0.5%−5.9% of the general US population, 8%−18% of psychiatric outpatients and 6.5%−42.7% of psychiatric inpatients.
14–18 BPD is highly comorbid with mood (50.9%), anxiety (59.6%), substance use (50.7%), and other personality disorders (73.9%).
14,15,17 The rate of co-occurring PTSD in individuals with BPD is approximately 30% in community samples.
15,16Although more women are diagnosed with BPD than men, recent research suggests this may be due to sampling (e.g., clinical populations) and self-report biases rather than a true difference in incidence.
19 In an epidemiological sample (gender ratio representative of a community population), men self-reported more BPD symptoms than women, whereas no difference was found between genders by informant report.
19 At the criterion-level, prevalence of some features of BPD (e.g., efforts to avoid abandonment, identity disturbances, impulsivity) varied by gender and assessment technique. Intense anger was endorsed more often by men through both self and informant report. In another study, self-report was found to be the better assessment for experiential features (e.g., identity disturbance, chronic emptiness), whereas a clinical interview was more reliable for observational or behavioral features (e.g., self-harm, impulsivity).
20The belief that individuals with BPD are unlikely to improve lingers, although there are now decades of evidence to the contrary. In the 1980s, several 15-year follow-up studies provided evidence of significant improvement of symptoms among individuals with BPD.
21 A recent systematic review of longitudinal studies reported that remission rates range from 33% to 99% and recurrence rates range from 10% to 36%.
22 Studies with follow-up periods longer than ten years reported higher rates of remission.
22 Lower rates of recurrence were seen after longer periods of remission.
22 Remission was also found to be common in a very large (10,786 individuals with BPD) retrospective (1995 to 2012) population based study, even when comorbid conditions remained active.
23 Some studies indicate that behavioral symptoms of BPD (e.g., impulsivity) remit at a faster rate than affective experiences and personality traits associated with BPD.
20,24–26 Similar rates across criteria have also been reported.
1 A study that categorized symptoms as either acute (e.g., NSSI, affective instability) or temperamental (e.g., chronic anger, intolerance of aloneness) reported that acute symptoms had higher remission and lower recurrence rates than temperamental symptoms at both 2-year and 4-year follow-ups.
27 A cross-sectional comparison of younger (age 18–25) and older (age 45–68) adults with BPD reported similar findings.
28 Older adults endorsed some diagnostic criteria less often (e.g., impulsivity, NSSI, suicidality, affective instability) and others more often (e.g., chronic emptiness) than the younger adults. Gaining understanding of the evolving presentation of BPD over the life span may assist clinicians in targeting specific symptoms for treatment and lead to greater overall improvement.
Negative perceptions of individuals with BPD among mental health care providers have potential to impact both access to care and quality of treatment provided.
29–33 For example, erroneous common beliefs (e.g., treatment resistance, need for resource intensive treatment plans) may create significant barriers to care.
33 Research indicates that more severe symptoms at baseline do not impair treatment response, and may actually promote better outcomes.
34 Thus, individuals with severe symptoms do not necessarily need more intensive or longer-term treatments. Some negative perceptions may be due, at least in part, to caseloads becoming enriched with less treatment responsive individuals (Berkson’s bias).
35 One set of studies found that simply providing participating clinicians with an inaccurate medical history containing a BPD diagnosis before viewing a video recorded clinical interview of a patient with panic disorder resulted in more negative clinical judgements (e.g., higher symptom ratings, lower optimism about treatment outcome).
36,37 Of particular importance, the patient did not exhibit any behaviors to support a diagnosis of BPD. This finding illustrates how an underlying negative stigma can impair clinician accuracy. Several groups have assessed the impact of incorporating methods of decreasing negative biases into clinical education. One day training workshops focused on specific treatments for BPD (Systems Training for Emotional Predictability and Problem Solving [STEPPS]; General Psychiatric Management [GPM]) have made measurable positive changes to clinicians’ beliefs about individuals with BPD (e.g., increased optimism, greater empathy).
38,39 These studies provide evidence that negative biases can be changed. Education on these and other effective treatments may also help combat the lingering belief that BPD is untreatable.
BPD differs from many other psychiatric disorders in that psychotherapy, not pharmacotherapy, is considered the first-line treatment. Medication-based interventions have shown at most moderate efficacy, and are typically used to treat co-occurring specific symptoms (e.g., depressed mood) rather than the disorder itself.
9,40,41 Several psychotherapeutic treatments have been developed specifically for BPD.
9,42 These treatments stem from major theoretical orientations including psychodynamic, cognitive-behavioral therapy (CBT), and client-centered. Dialectical-behavior therapy (DBT) is the most widely used and well-studied treatment for BPD. A meta-analysis of randomized clinical trials (RCTs) found that only DBT and psychodynamic were clearly more effective than comparison conditions.
43 Other interventions with research support include transference-focused psychotherapy (TFP), mentalization-based treatment (MBT), schema-focused therapy (SFT), and STEPPS.
9 The length of these treatments and large amount of required resources (e.g., clinician training, materials, provision of individual and/or group treatment, case management) reduces their availability.
9 However, newer evidence-based-treatments are cost effective (i.e., decreased overall mental health and community costs) compared with no treatment or treatment as usual.
44 GPM was created as a less intensive comparison treatment for a large RCT of DBT.
45 Contrary to expectations, GPM was found to be as effective as DBT over 12 months of treatment and at 24 months follow-up.
9,45,46 Existing evidence on DBT indicates that much of the treatment gains patients experience occur within the first six months. Researchers are now examining which components of the treatment are most effective in order to understand whether treatment gains can occur with fewer resources. Studies investigating GPM and recent dismantling studies of DBT provide evidence that treatment may require considerably fewer resources than previously assumed.
9,45–47 This has the combined benefits of improving accessibility for patients and decreasing burden on clinicians.
While the majority of individuals with BPD eventually experience symptom remission, functional improvement is less common.
1,2,48,49 In one longitudinal study, recovery was defined as no longer meeting diagnostic criteria for the disorder (symptom remission) and a Global Assessment of Functioning Scale (GAF) of at least 61, indicating a generally good functional outcome.
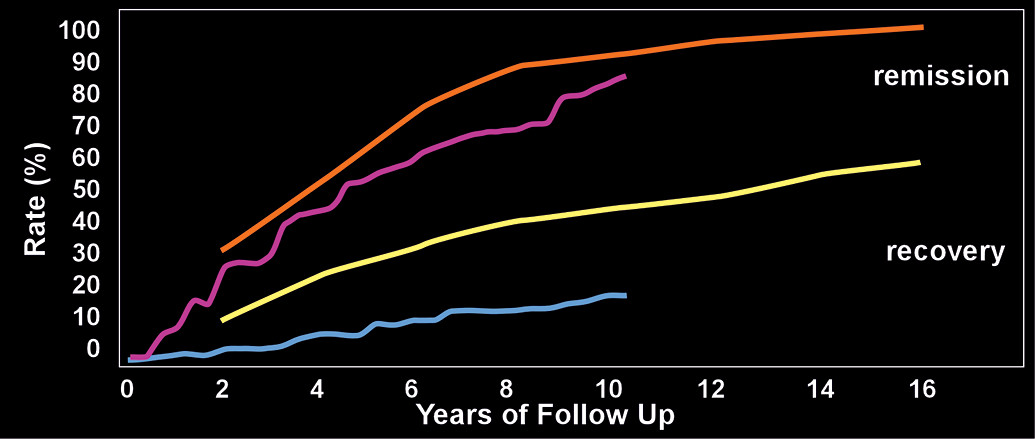
2,48 All participants were initially inpatients. By the end of the 16-year follow-up (data collected every 2 years), the cumulative remission rates ranged from 78% for eight years of sustained remission to 99% for two years of sustained remission (
Figure 1).
2,48 Recovery occurred less frequently, ranging from 40% for eight years of sustained recovery to 60% for two years of sustained recovery (
Figure 1). A 10-year follow-up study that recruited from both inpatient and clinic populations reported a 91% cumulative rate of remission sustained for at least one year (
Figure 1).
1 The 10-year rate of relapse was 11%.
1 There were only modest functional improvements, as no individuals had an initial GAF score of at least 70; whereas 21% had reached this mark by 10 years (
Figure 1). Another study did a 10-year follow up on individuals with BPD who had previously participated in a clinical trial.
49 Over half (55%) were considered to be in remission. However, the only aspect of functioning that was significantly improved was living arrangements. Initially 47% were living with their parents, while 10 years later 88% were living on their own or with a partner. A recent study compared individuals with and without BPD recruited from the community to individuals with BPD recruited from clinical venues (e.g., outpatient, residential).
50 Community-based individuals with BPD were less likely than individuals without BPD to experience good overall functioning, but were also less likely to experience the more severe impairments seen in the clinically-based individuals with BPD.
50 Although substantial functional impairment typically lingers when symptoms remit, some individuals with BPD are eventually able to achieve a full recovery. Improved understanding of the underlying neurobiology of this disorder may provide insights into the causes of sustained functional impairments across the lifespan of individuals with BPD, enabling creation of new treatments or evolution of existing ones.
Dysregulation of emotion and impulsivity are considered core deficits in BPD. The proposed neural correlates are limbic (e.g., amygdala) hyper-reactivity and prefrontal (e.g., dorsolateral) hypo-reactivity. However, as noted in a recent review, both behavioral and functional magnetic resonance imaging (fMRI) studies have provided only modest supporting evidence.
51 The most recent meta-analysis of fMRI studies (2001 to 2014, 18 studies) comparing BPD and healthy control groups during processing of negative (compared with neutral) emotional stimuli reported multiple areas of difference.
52 Of note, increased activation in the area of the amygdala and hippocampus (left side only) were specific to studies of medication free BPD groups.
52 This was not replicated by a recent study in which no areas were significantly different between the medication free BPD and control groups when overt exposure to negative faces was compared with neutral stimuli.
53 Increased activation in the area of the left amygdala and hippocampus (and other areas) was found only when overt exposure to negative faces was compared with the fixation condition. In contrast, covert exposure to negative faces evoked greater activation in multiple areas when compared with either the neutral or fixation conditions.
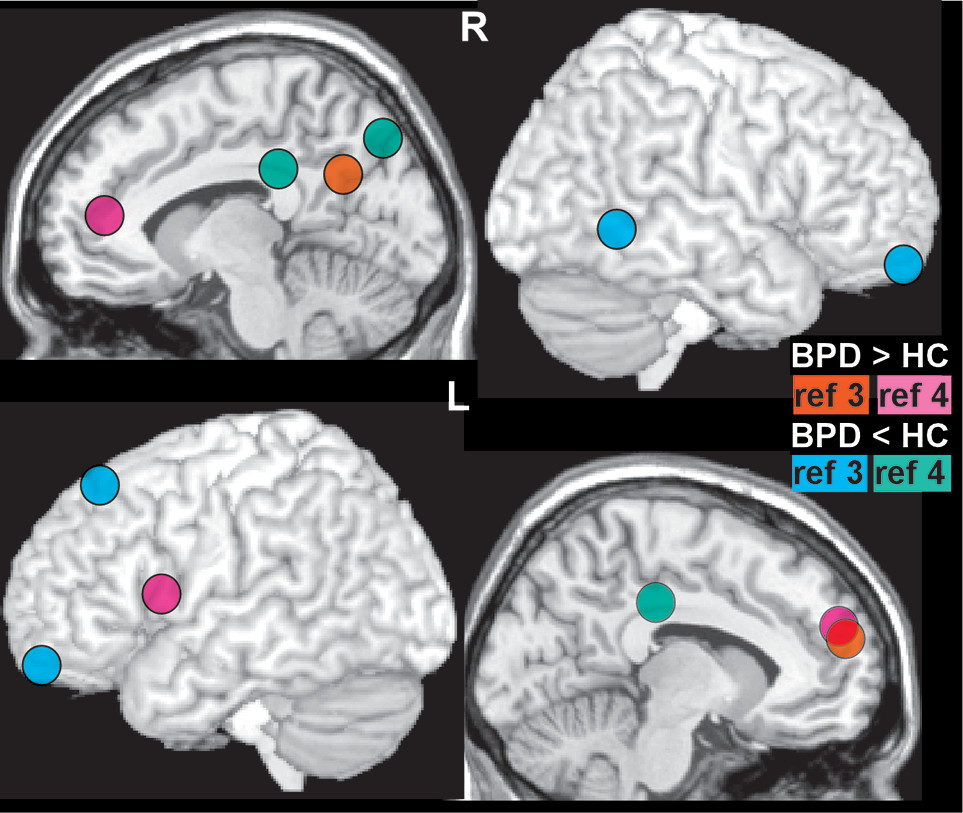
53 Establishing neural correlates of altered functioning in BPD is complicated by multiple factors including the possibility of baseline (resting state) differences. A meta-analysis that combined across resting state fMRI and positron emission tomography (PET) studies reported modest evidence for regional differences between BPD and control groups (
Figure 2).
3 Of note, the fMRI and PET metrics had little anatomic overlap (Cover). While meta analytic approaches are valuable for identifying findings that are robust across studies, specifics of study selection are critical. A replication meta-analysis that changed two decisions (replaced one study with another from the same group, included one study that had been excluded) reported mostly different results (
Figure 2).
4Recent reviews and meta analyses of studies comparing accuracy on various facial emotion recognition tasks between groups with BPD and healthy individuals indicate all possible outcomes (better, worse, same).
54–56 Although not found in all studies, misclassification of neutral faces as negative by individuals with BPD has been the most consistently reported difference.
54–56 This has also been found in early onset and first episode psychosis, and thus cannot be considered specific to BPD.
57,58 As noted in one meta-analysis, even when present the difference is relatively small, suggesting at most a subtle impairment in emotion recognition.
54 Consistent with this, a recent study utilizing a battery of five tests of Theory of Mind (ToM) reported that performance of individuals with BPD (most on psychotropic medications) was similar to healthy controls on all but the most complex tasks.
59 The groups did not differ on overall accuracy in identifying mental states (Reading the Mind in the Eyes Task), identifying or attributing emotions (Expression Attribution Test) or in correctly attributing mental states to understand others’ behaviors (False Belief Picture Sequencing Task). The BPD group performed more poorly on the jokes that required ability to understand the cartoon character’s false beliefs or ignorance (Joke Appreciation Task). This did not correlate significantly with clinical scales. The BDP group was also less able to perform the most complex ToM task (Faux Pas Test), with the greatest differences between groups on questions assessing understanding of affective (e.g., who was hurt) and cognitive (e.g., why the faux pas occurred) aspects. However, there was no difference in correctly identifying the false belief. The authors note that this pattern of results suggests that participants with BPD were less likely to spontaneously take the empathic perspective in order to understand the other’s mental state. They were able to accurately identify the false beliefs when prompted.
59Decreased trust and increased negative perceptions in social situations have been proposed to contribute to the impaired interpersonal functioning that is considered central to BPD.
60 A recent review of game-theoretical studies noted that individuals with BPD cooperated significantly less than healthy controls on a tit-for-tat strategy game (Iterated Prisoner’s Dilemma), although cooperation rates did not correlate with either trait impulsivity or hostility.
61 Results from an iterative investment game (Trust Game) were consistent with individuals with BPD having lower trustworthiness and higher expectations of negative reciprocity. BPD groups were also less likely than the control groups to reject offers (Ultimatum Game). The authors note that the overall pattern for individuals with BPD is closer to a purely rational approach to economic decision making, much less modulated by social expectations of cooperation or reciprocity than is the norm.
61 Although commonly attributed to impairments in social cognition, this pattern of behaviors is also consistent with viewing the world as highly unpredictable and other people as mostly untrustworthy.
61,62 This interpretation suggests that interventions directed toward facilitating recognition of overly negative expectations and development of more accurate perceptions of events and other people might be of benefit.
61,62Impulsivity and heightened emotional reactivity have been proposed as key aspects of BPD that contribute to poor decision making. A meta-analysis of studies utilizing decision making tasks reported that BPD was associated with steeper discounting for delayed rewards but reversal learning was not impaired.
63 Although a modest increase in poor decision making (Iowa Gambling Task) was present overall, this was strongly influenced by gender and medication status.
63 An fMRI study comparing two groups of patients with BPD (no psychotropic medication within two weeks) to healthy individuals on tasks requiring response inhibition reported no consistent differences in either actual performance or patterns of brain activation.
64 A series of studies from the same research group compared BPD to healthy groups on multiple tasks probing aspects of impulsive responding prior to and following induction of acute stress.
65–67 On self-report measures individuals with BPD had higher baseline levels of anger, aggression and impulsivity than healthy controls and all measures were further elevated by stress. However, performance of the groups did not differ either at baseline or after stress induction on aggressive responding (Point Subtraction Aggression Paradigm), response cancellation (GoStop Task), or feedback informed decision making (Iowa Gambling Task). Although performance on response inhibition (Immediate Memory Task) did not differ between groups at baseline, the BPD group had a significantly higher failure rate than the control group after stress induction. The BPD group’s higher preference for immediate rewards (Delay Discounting Task) at baseline was not altered by stress induction. Similarly, a study utilizing BPD-relevant negative pictures to induce emotional stress found no differences between the BPD (most on psychotropic medication) and healthy control groups in emotional reactivity as measured by either physiological metrics (heart rate, respiratory sinus arrhythmia, electrodermal activity) or self-report measures.
68 There were baseline differences in physiological metrics (higher heart rate, lower respiratory sinus arrhythmia) indicating lower resting parasympathetic tone in the BPD group. This agrees with a recent meta-analysis that reported BDP groups differed significantly from healthy groups in resting state cardiac vagal (parasympathetic) tone, as indicated by lower values for respiratory sinus arrhythmia and/or vagally mediated heart rate variability.
69 Heart rate variability has been proposed as a psychophysiological indicator of capacity for emotion regulation and inhibitory control.
69 These findings suggest that individuals with BPD must deploy greater emotion regulatory efforts in order to keep emotional reactivity properly controlled. This indicates that strengthening of related skills (e.g., mindful awareness, distraction strategies, anger management) could be an important therapeutic target.
65A major confounding influence across studies of BPD is heterogeneity in symptom presentation (i.e., 256 possible symptom combinations).
70 Multiple other factors may also be contributory. A recent systematic review of the evidence for altered interpersonal functioning in BPD (i.e., social cognition, reactivity to interpersonal stressors, interpersonal aggression, differences in trust and cooperation) emphasized the importance of utilizing objective assessments of behavior rather than relying on self-report measures.
60 Additional factors noted as having the potential to contribute to mixed findings across studies included the influences of gender, medication status, comorbid conditions, trauma history, and individuals’ state (e.g., arousal, mood, dissociation) at the time of evaluation.
51,55,60,71,72A few studies have begun to address the confounding influence of BPD’s symptomatic heterogeneity by investigating effects of individual differences in specific BPD symptom domains on level of task-related fMRI activations. One study assessed the influence of affective instability (Affective Lability Scale, ALS) and emotional regulation (Difficulties with Emotion Regulation Scale).
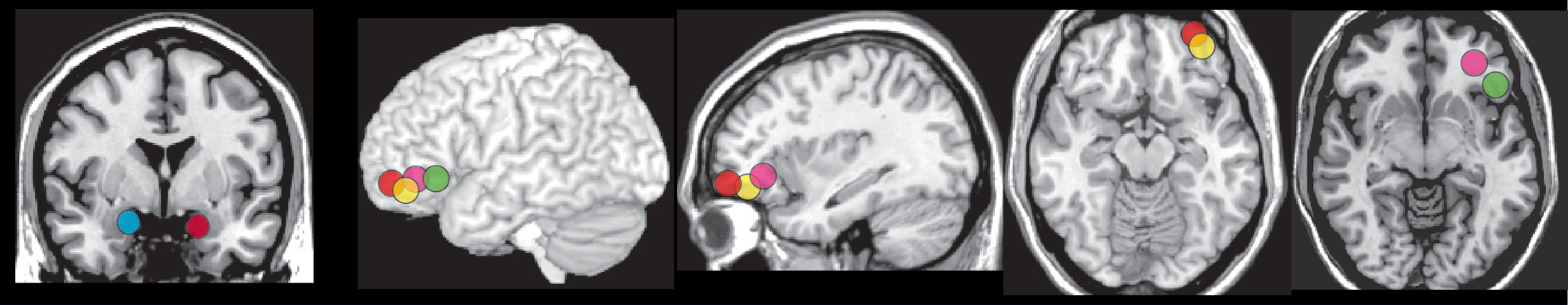
5 Higher affective instability was positively correlated with left amygdala activation during immersive viewing of emotionally aversive (compared with neutral) images (
Figure 3).
5 In contrast, greater difficulty with regulating emotions was negatively correlated with ventrolateral prefrontal cortex activation while employing distancing to decrease negative emotions (compared with immersive viewing) during viewing of emotionally aversive images.
5 Another study assessed the symptom domains of impulsivity (Barratt Impulsiveness Scale) and aggression (Lifetime History of Aggression).
73 Higher impulsivity was positively correlated with greater activations within multiple areas of prefrontal cortex (dorsolateral, medial, orbitofrontal) and caudate during the negative valence trials (compared with positive valence trials) of an affective Go/NoGo task (emotional faces).
73 In contrast, higher aggressiveness was negatively correlated with greater activations in orbitofrontal cortex, putamen and hippocampus. A longitudinal pilot fMRI study of transference focused psychotherapy for individuals with BPD utilized an affective Go/NoGo task (emotional words) to identify changes in activations that correlated with improvements on specific symptom domains.
6 Although task performance was not altered by treatment, there were multiple areas in which activations changed (both increases and decreases) posttreatment during negative valence trials (compared with neutral). Improvements on affective instability (ALS) correlated with decreased activation in right amygdala/parahippocampal cortex (
Figure 3) and increased activations in left posterior orbitofrontal cortex/ventral striatum. Improvements on impulsivity (Multidimensional Personality Questionnaire constraint score) correlated with increased activation in left anterior cingulate cortex. Improvements on aggression (Overt Aggression Scale) correlated with increased activation in the left inferior frontal gyrus (trend level) (
Figure 3).
6 Two studies that grouped individuals with BPD by presence/absence of a history of self-harm (suicide attempts; nonsuicidal self-injury, NSSI) identified differences in neural activations in the absence of differences in task performance.
7,8 A history of multiple suicide attempts (compared with no suicide attempts) was associated with greater activation in left lateral orbitofrontal cortex during both immersive viewing and while employing distancing to decrease negative emotions during recall of aversive (compared with neutral) personal memories (
Figure 3).
7 A history of multiple NSSI episodes (compared with no NSSI episodes) was associated with greater activation in left lateral orbitofrontal cortex to unexpectedly high gain trials on a betting task (
Figure 3).
8 As noted in all of these studies, the results support the possibility of different neural substrates for at least some of the BPD symptom domains. While all of these findings must be considered preliminary, they support the potential value of incorporating individual differences in clinical symptomology in order to better characterize the neural correlates of BPD.
In summary, there is now a strong evidence base that BPD often eventually remits and that some patients can achieve a functional recovery. The number of studies examining the neural underpinnings of BPD has increased tremendously in the past two decades. There are, however, many limitations associated with the existing studies. Significant confounders include the vast symptom heterogeneity of the disorder, symptom overlap with other clinical disorders, and the high level of comorbidities. This disorder is associated with high levels of risky behavior, functional impairment, and mental health treatment utilization. Understanding more about the underlying neural mechanisms of BPD may lead to more targeted, streamlined, and effective treatment strategies.