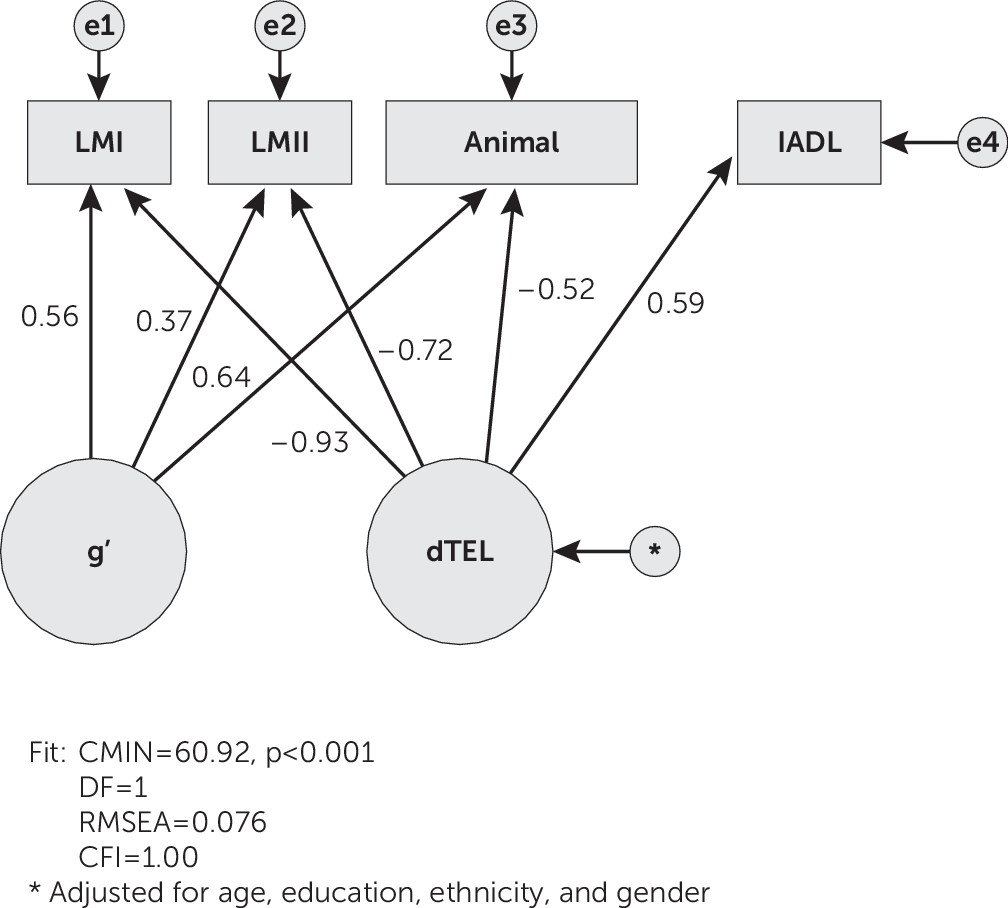
“δ” (for “dementia”) is a latent phenotype of dementia itself, as distinct from impairment(s) in domain-specific cognitive performance. δ’s confirmatory bifactor model explicitly extracts the fraction of cognitive performance variance that is shared with one or more measures of functional status (i.e., in a structural equation model [SEM] framework). δ is relatively free of measurement error, continuously distributed, strongly related to Instrumental Activities of Daily Living (IADLs),
1 agnostic to dementia’s etiology,
2,3 and it can be modeled in almost any cognitive battery that also includes a measure of IADL. Thus, we further distinguish between δ (i.e., “the cognitive correlates of functional status”) and “d” (i.e., δ’s reification as a composite score in a specific cognitive battery or analysis).
δ’s bifactor model defines it as a subdivision of “general intelligence,” as operationalized by Spearman’s “
g” factor (as opposed to observed “IQ” tests).
4 Spearman showed that
g is “indifferent to its indicators.” δ shares this property. It can be constructed from almost any ad hoc combination of cognitive and functional status measures (i.e., by confirmatory factor analysis in SEM). Each d composite is therefore likely to be calculated from a unique set of indicators. Nevertheless, they all estimate δ and are homologous to each other regardless of their indicators. We therefore refer to each d composite as a δ “homolog.” Because δ is “indifferent to its indicators,” δ homologs can be engineered to satisfy any agenda (e.g., brevity, availability in translation, administration via the Internet, etc.).
In the present article, we describe a new δ homolog, “dTEL,” derived from a brief cognitive battery that could be administered over the telephone by lay psychometricians. dTEL might allow for the accurate assessment of dementia severity, regardless of the patient’s location and in the absence of an experienced clinician.
Discussion
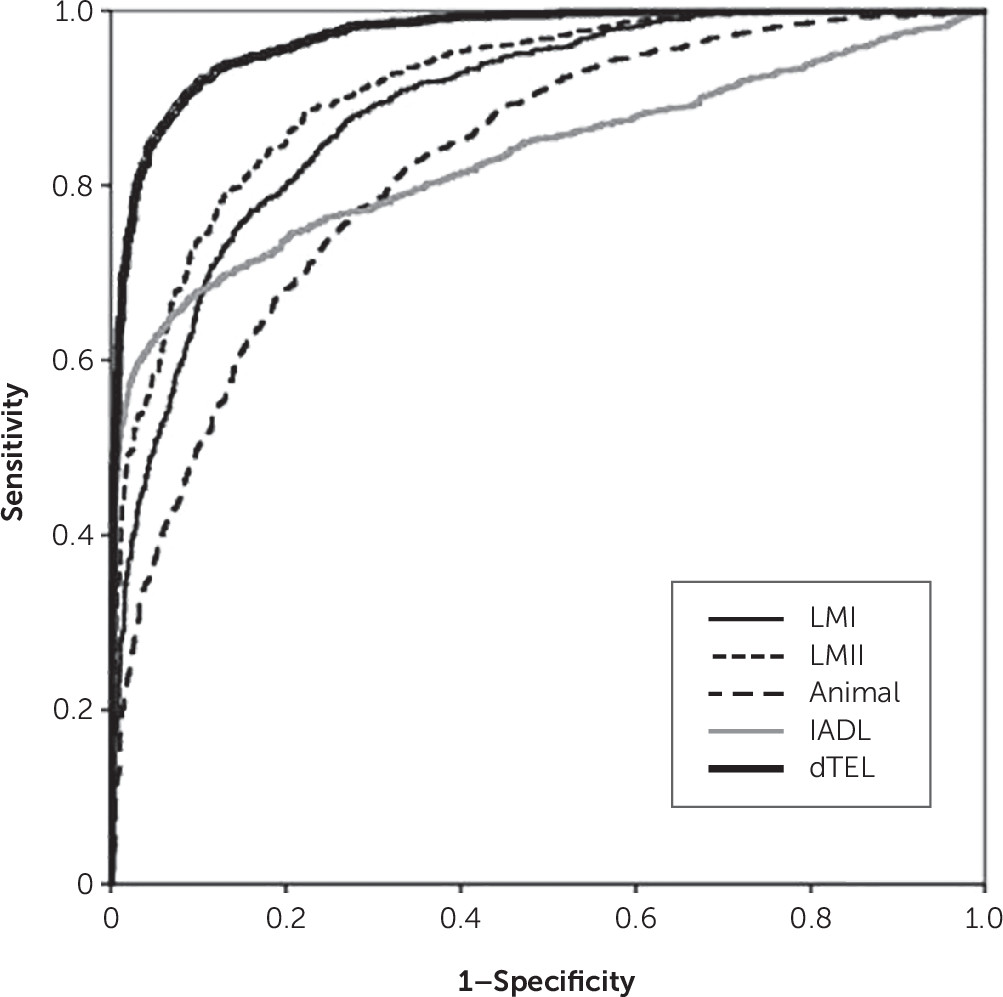
In this analysis, the latent variable δ was constructed from a set of verbal cognitive measures. Two features of this model are remarkable. First, to our knowledge, this is the twelfth δ homolog constructed to date. Regardless of their indicators, all have exhibited strong associations with IADL and/or CDR scores, as well as high AUCs for the discrimination between AD patients and control subjects.
2,27–33 Second, the indicators for this model could be easily obtained over the phone, possibly by paraprofessional psychometricians and/or mid-level care providers.
dTEL’s AUC was higher than those of any of its observed indicators. This helps justify the administration of dTEL’s battery relative to any single dTEL indicator. This finding supports our claim that d homologs necessarily perform “greater than the sum of their parts,” regardless of whether they are constructed from comprehensive psychometric batteries,
28,30 brief screening batteries (as in this example), or the item sets of individual measures.
28,31 Similarly, the AUC we achieved here is superior to those reported for other telephone-administered psychometric measures.
34–37We previously validated a brief d homolog constructed from the 5-item Telephone Executive Assessment Scale (TEXAS).
31,38 The dTEXAS homolog had an AUC of 0.92 (CI=0.880–0.968) for the discrimination between NCs and AD patients, superior to the entire 25-item Executive Interview,
39 from which the TEXAS is derived.
31 While both dTEL and dTEXAS might be administered over the phone, dTEXAS’s ease of administration must be weighed against its significantly poorer diagnostic accuracy.
Like dTEL, dTEXAS correlated strongly with IADL (r=−0.861, p<0.001).
31 Either composite can be constructed from its cognitive indicators alone and used to predict IADL (i.e., δ’s “target” indicator). The use of such “restricted” composites frees d homologs from the need for an intact patient-caregiver dyad. dTEXAS’s restricted composite correlated with IADL (r=−0.51, p<0.001). A restricted dTEL composite might also be deployed among subjects without informants.
δ recasts dementia as “the cognitive correlates of functional status.” Our approach converts dementia from a category to a continuous dimension. The dementia severity of any two persons can thereby be compared, even persons performing within the normal range on δ’s indicators. Even small changes in the d-scores of persons without dementia appear to increase the risk of prospective conversion to clinical dementia.
29 By using this method, the progress toward dementia in a patient without dementia can be followed over time, or in response to intervention.
dTEL’s validity outside of clinical AD has yet to be determined. Although TARCC purports to be a study of “AD,” an estimated 20% of patients diagnosed with clinical “AD” by experienced clinicians may be without beta-amyloid as determined with positron emission tomography.
40,41Moreover, δ homologs appear to be “agnostic” to the etiology of dementia. Two recent studies (by an independent group of investigators) confirm that δ is agnostic to dementia’s etiology. Both were conducted with the National Alzheimer’s Coordinating Center’s Unified Dataset (N=26,000). Gavett et al.
2 found δ’s AUC to be 0.96 for the discrimination between individuals with dementia of any etiology (i.e., AD, Lewy body disease, fronto-temporal dementia, and “vascular dementia”) and individuals without dementia (patients with mild cognitive impairment and control subjects). δ also confirms the disabling and therefore inherently “dementing” cognitive effects of depression.
42 John et al.
3 demonstrated that δ scores are unable to distinguish between any combination of dementing conditions. These findings suggest that clinical dementia arises from the sum of independent δ-related processes, regardless of etiology.
Since δ is essentially the sole cognitive determinant of dementia severity, both cross-sectionally (e.g., r=0.99 versus the CDR-SOB) and longitudinally (e.g., r=0.94 × ΔCDR-SOB),
2,43 it seems likely that an optimal dTEL diagnostic threshold could be applied to a wide range of potentially dementing conditions (e.g., traumatic brain injury). Whether this can be accomplished over the telephone by lay psychometricians awaits demonstration.
The advantages of dTEL may be incrementally superior to that of its alternatives. Results similar to ours have been achieved by other measures. Manly et al.
44 reported the diagnostic accuracy of telephone screening with the Telephone Interview for Cognitive Status (TICS)
45 and the Dementia Questionnaire (DQ)
46 in a large, ethnically diverse community sample (33.7% Hispanic, i.e., similar to TARCC). The TICS demonstrated an AUC of 0.94 for discrimination between participants with and without dementia (sensitivity=0.88, specificity=0.87). The DQ did less well (i.e., AUC=0.84, sensitivity=0.66, and specificity=0.89, respectively). In the Alzheimer’s Disease Anti-inflammatory Prevention Trial,
47 the TICS group did not perform as well (i.e., sensitivity=0.73 and specificity=0.95). Instead, it took a telephone assessment battery, composed of five measures, including the TICS, to achieve a comparable diagnostic accuracy (i.e., sensitivity=0.83 and specificity=0.82 for the diagnosis of dementia). That battery’s administrative burden would be similar to that of dTEL.
Regardless, any of the above assessments might be reengineered as δ homologs and thereby improve upon their diagnostic accuracy. The diagnostic accuracy, demonstrated by multiple δ homologs to date, suggests that a wide range of δ homologs might be engineered to meet the needs of a wide range of users and in a wide range of settings. δ homologs might be engineered for their brevity, low cost, availability in translation, or mode of administration (e.g., telephone, computer, website, etc.). Dementia might then be diagnosed far from memory clinics, without expert adjudication and by a larger range of professionals.
In summary, the dTEL homolog achieves a very high AUC for discrimination between AD patients and control subjects. It offers the potential convenience of administration by telephone. dTEL, or similar δ homologs, might allow high-quality dementia diagnoses to be made in minority or rural populations, far from tertiary care centers and without expert adjudication.