Traumatic brain injury (TBI) is a major public health problem, with an annual incidence rate of 2.5 million in the United States (
1). Major depression is a common sequela in patients with TBI, with rates of approximately 50% (
2–
4), compared with the general population in which the lifetime prevalence of major depressive disorder is about 19% (
5). Accordingly, depression following TBI (i.e., “TBI depression”) is a significant health concern that warrants clinical attention.
TBI depression is characterized by persistent sadness, anhedonia, feelings of worthlessness, hopelessness, loss of interest in work and family activities, poor motivation, decreased social contact, and suicidal thoughts. These symptoms start after TBI, occur at least several days per week, last for 2 weeks or more, and significantly interfere with day-to-day functioning (
6). There is some evidence to suggest that the rate of suicidality is rather substantial with TBI depression. In a study that compared patients with TBI of all severities (N=105) with matched healthy control subjects (N=74), suicidal ideation was present in 33% of the TBI cohort compared with 1.4% of the healthy control cohort (
7). In addition, there appears to be a negative effect from the depression on the recovery process, with TBI symptoms being more persistent when accompanied by depression (
8).
Multiple factors may play a role in the development of TBI depression, including preinjury factors (such as pre-TBI psychiatric history, poor psychosocial functioning, and alcohol misuse), injury factors (such as injury severity and injury to the left dorsolateral frontal lobe), and postinjury factors (such as persistence of physical symptoms following TBI, psychosocial dysfunction, and poor social support) (
9). Jorge et al. (
10,
11)reported that development of depression in the early recovery period following TBI may be more strongly related to injury factors, while late-onset depression may be more related to psychosocial factors. In a recent study, Singh et al. (
12) reported that TBI depression can be associated with demographic and clinical variables, such as nonwhite ethnicity, female gender, alcohol intoxication at injury, positive findings on computerized tomography scans, and inability to return to full-time work. TBI depression is most common in the first year after TBI, although it can occur anytime thereafter (
13).
Factors associated with prolonged persistence of neuropsychiatric disorders following TBI include pre-TBI psychiatric history, previous TBIs, gender, age, personality style, poor social support, inadequate treatment, and ongoing litigation or compensation issues (
14,
15). In a study on the persistence of psychiatric symptoms after TBI, Silver (
16) observed that delayed-onset neuropsychiatric symptoms are more likely to have a nonneurological component, but may not be unrelated to the original injury. It is not always possible to directly associate the TBI event with neuropsychiatric symptoms because the acute event may not be followed by a chronic injury detectable by objective methods. He hypothesizes that the initial TBI event may cause ‘cognitive dysfunctional feedback loops’ that are associated with the persistence of symptoms; it may be that TBI can initially trigger a cascade of symptoms that eventually takes on a life of its own.
Despite its high prevalence and the socioeconomic burden of TBI depression, there are only a few controlled treatment trials of the disorder (
17–
19), with only one (
17) of these studies favoring antidepressants compared with placebo. However, in a recent systematic review and meta-analysis conducted by Salter et al. (
20) to determine the effectiveness of pharmacotherapy for the treatment of TBI depression, antidepressant pharmacotherapy was associated with a large, favorable treatment effect (Hedges g=1.169, 95% CI, 0.849–1.489, p<0.001) based on data available for pooled analysis of within-groups treatment effect from eight studies. Similarly, in focusing the analysis on only placebo-controlled trials, treatment with antidepressants was associated with statistically significant reduction in depressive symptoms (standardized mean difference=0.84, 95% confidence interval, 0.314–1.366, p=0.002). Thus, the data have been inconsistent in showing statistically significant differences between active treatment and placebo, perhaps due to small sample size and lack of adequate power to capture differences.
The present pilot study was designed to determine the effect size of repetitive transcranial magnetic stimulation (rTMS) for the treatment of TBI depression. There are some advantages to rTMS over medications. Specifically, rTMS may lend itself to the diffuse neuropathology of TBI more than pharmacotherapy, since unlike pharmacotherapy, rTMS is known to induce widespread neuromodulation and strengthen synaptic connections in the fronto-subcortical-limbic circuitry (
21–
23). Additionally, rTMS does not affect multiple parts of the body, unlike pharmacotherapy, and thus is focal in a sense as well. Another form of neuromodulation, ECT, has also been studied for TBI depression. A retrospective, uncontrolled study of ECT showed improvement in TBI depression in nine out of 11 study subjects with no decline in cognitive function (
24). However, unlike ECT, rTMS does not require general anesthesia and does not normally induce seizures. Therefore, it may potentially have a better side-effect profile.
There are limited published studies in the literature on the use of rTMS for treating TBI depression. Three studies have been identified: a case report by Fitzgerald et al. (
25), a case report by Nielson et al. (
26), and a randomized trial by He et al. (
27) comparing rTMS and a tricyclic antidepressant with a tricyclic antidepressant alone. All three studies reported positive effects of rTMS on depressive symptoms. In studies of rTMS for the treatment of medication-resistant idiopathic major depression, there is burgeoning support demonstrating it to be superior compared with sham treatment, as both monotherapy and adjunctive to antidepressant treatment (
28–
31).
Abnormally increased activity and connectivity between regions of the medial prefrontal cortex (PFC), such as Brodmann’s area 25, have been postulated in the etiology of major depression (
32,
33). High-frequency left-sided (HFL) rTMS over the dorsolateral prefrontal cortex (DLPFC) has been shown to increase cortical excitability locally and decrease hyperconnectivity within the medial PFC with improvement in depressive symptoms (
34). Even though the mechanism of improvement in depressive symptoms with low-frequency right-sided (LFR) rTMS over the DLPFC is not clearly known, electrophysiological and functional neuroimaging studies have shown reduced cortical excitability with LFR rTMS (
35,
36), and clinical studies have shown that LFR rTMS can be effective for depressive symptoms (
37,
38). Clinical studies have also demonstrated antiepileptic effect with LFR rTMS (
39), in contrast to risk of seizures with HFL rTMS. Other advantages of LFR over HFL rTMS include greater tolerability, less localized discomfort at the stimulation site, reduced stimulation of the facial nerve, and fewer headaches (
40,
41).
Given this background, it is clear that there is a need to expand the range of available treatments for TBI depression and determine other treatment strategies that are comparable or superior to currently available antidepressants. There is preliminary evidence, albeit rather limited, that rTMS may be a viable option.
We conducted the present study to acquire data that will inform 1) the safety and tolerability of LFR rTMS in patients with TBI compared with sham treatment and 2) the effect size of LFR rTMS over the DLPFC on TBI depression and common comorbid psychiatric symptoms. This treatment strategy has the potential to directly target the brain regions involved in mood regulation and therefore may provide equal or better efficacy compared with antidepressants while causing minimal side effects.
Methods
Study procedures were approved by the institutional review boards at Johns Hopkins Medicine and the Department of Defense. Written, informed consent was obtained from all study subjects, and the study was HIPAA compliant. Participants were recruited from several sources, including the Brain Injury Clinic at the Johns Hopkins Bayview Medical Center, referral from other Johns Hopkins outpatient clinics, and advertisements placed in local newspapers and via social media. Adults aged ≥18 years who met Department of Defense criteria for traumatic brain injury and Structured Clinical Interview for DSM-IV (SCID-IV) criteria for major depressive disorder and had a score >10 on the 17-item Hamilton Depression Rating Scale (HAM-D) were eligible to participate. Exclusion criteria included current antidepressant use (to qualify for the study, individuals had to be off antidepressants or be willing to taper off by working with their treating physician); medical instability; active substance abuse ≥1 month prior to study entry; psychosis; personality disorder of sufficient severity that it could interfere with study participation; frontal lesion(s) on screening MRI brain scan due to any cause; bipolar disorder; progressive dementia; Mini-Mental State Examination (MMSE) score ≤24; positive and unmitigated response to any question on the Transcranial Magnetic Stimulation Safety Screen questionnaire; ECT within 6 months prior to the screening visit; history of treatment with rTMS therapy for any disorder; history of treatment with vagus nerve stimulation; history of treatment with deep brain stimulation; cardiac pacemakers, implanted medication pumps, or intracardiac lines; intracranial implant or any other metal object within or near the head; implanted neurostimulators; known or suspected pregnancy; and history of seizures, posttraumatic epilepsy, or any other neurological disorder that could increase the risk of seizure.
The inhibition versus excitability component of LFR rTMS was particularly important because of the increased risk of seizure with excitation of cortical regions. We chose the inhibition over the excitability component of rTMS to minimize the risk of seizures. Although the risk of seizure from rTMS is very low, and the risk of first seizure in the late postinjury period following mild or moderate TBI is also relatively low (
42), we took a conservative approach to treatment selection in order to reduce the risk of rTMS-related seizures. We also excluded persons with posttraumatic epilepsy, frontal lesions, and other neurological disorders that could increase the risk of rTMS-related seizures.
In order to enhance the accuracy of effect size determination for LFR rTMS over the DLPFC for the treatment of TBI depression and common comorbid psychiatric symptoms, concurrent treatment with antidepressant medications was not permitted. Individuals receiving such treatments were excluded from study participation. In addition, individuals on the investigative team, personnel affiliated with the study, and their immediate family members were excluded from study participation.
After obtaining informed consent, all potential study subjects underwent baseline assessment for eligibility to participate in the study. All eligible individuals underwent a comprehensive neuropsychiatric evaluation, including collection of demographic information, TBI history, and SCID-IV criteria met to establish the diagnosis of major depressive disorder and other axis I psychiatric disorders. Following baseline assessment, participants were randomly assigned 1:1 to either rTMS intervention or sham treatment, stratified by time since injury (<5 years versus ≥5 years) using custom software developed by the study statistician (JL). All study subjects had five follow-up assessments. The first took place within a week of receiving the 20th rTMS or sham treatment. Subsequent follow-up evaluations took place 4, 8, 12, and 16 weeks after the 20th intervention.
Assessments
The 17-item HAM-D was used to assess the severity of depression, which was the predetermined primary outcome measure of the study. There were several secondary outcome measures, including the Clinical Global Impression-Severity (CGI-S) scale, used to assess severity of illness, the Clinical Global Impression-Improvement (CGI-I) scale, used to assess global improvement, and the Beck Scale for Suicide Ideation (BSSI), used to assess attitudes toward suicide.
Comorbid neuropsychiatric symptoms were also assessed with other well-validated scales. These measures included the 7-item Generalized Anxiety Disorder (GAD) scale, used to assess the severity of anxiety; the Davidson Trauma Scale (DTS), used to assess the severity of posttraumatic stress symptoms; the Pittsburgh Sleep Quality Index (PSQI), used to assess the presence and severity of subjective sleep problems; the Epworth Sleepiness Scale (ESS), used to measure daytime sleepiness; the Neurobehavioral Rating Scale (NBRS), used to assess various neuropsychiatric symptoms; the Rivermead Post-Concussion Symptoms Questionnaire (RPQ), used to assess postconcussive symptoms; and the Fatigue Severity Scale (FSS), used to assess fatigue severity. Other instruments included the General Medical Health Rating Scale, used to assess overall medical comorbidity; the Social Ties Checklist (STC), used to assess the presence or absence of social connections; and the Satisfaction With Life Scale (SWLS), used to determine overall satisfaction with life.
In addition to standardized rating scales, study subjects also underwent a battery of neuropsychological tests. Neuropsychological tests administered included the Montreal Cognitive Assessment (MoCA), Trail-Making Test, Part A, Trail-Making Test, Part B, Controlled Oral Word Association Test, Wisconsin Card Sorting Test (WCST), Stroop Test, Brief Test of Attention, Hopkins Verbal Learning Test, and Brief Visual Memory Test (BVMT).
Participants were offered two overnight polysomnograms. One polysomnogram was conducted at baseline before the initiation of intervention (rTMS or sham), and the second was conducted during week 16 (after rTMS and sham stimulation concluded).
A subset of participants who were interested and willing also underwent standard MRI brain scans (T1-weighted, T2-weighted, fluid attenuated inversion recovery, and gradient echo susceptibility weighted sequences) with diffusion tensor imaging (DTI) sequences. MRI DTI scans were administered preintervention and postintervention (at the end of 16 weeks). DTI data were acquired with a single-shot echo-planar imaging sequence with sensitivity encoding, using a parallel-imaging factor of 2.5. The imaging matrix was 96×96, with a field of view of 212×212 mm (nominal resolution, 2.2 mm), zero-filled to 256×256 pixels. Transverse sections of 2.2-mm thickness were acquired parallel to the anterior commissure-posterior commissure line. A total of 60–65 sections covered the entire hemisphere and brainstem without gaps. Diffusion-weighted images at b=700 seconds/mm2 along 32 directions were acquired in addition to five additional images with minimal diffusion weighting (b=33 mm2/seconds). The scanning time per data set was approximately 4 minutes. To enhance the signal-to-noise ratio, this procedure was repeated twice. From this, fractional anisotropy (FA) and trace values were calculated.
We performed hypothesis-based regional analysis of the DTI-derived parameters using manual regions of interest (ROI). ROIs included anterior limb of the internal capsule, superior longitudinal fasciculus, body of the corpus callosum, cingulum white matter, superior frontal white matter, middle frontal white matter, and inferior frontal white matter. In addition, we performed whole-brain white matter analyses using a multiatlas segmentation technique (
43–
45) in which the white matter was segmented into 171 structures.
Intervention
Through the Brain Stimulation Program at The Johns Hopkins Hospital, rTMS intervention was delivered with a Magstim Super Rapid 2 stimulator with a focal double 70-mm air-cooled coil (Magstim Company Limited, Whitland, United Kingdom). Control subjects received treatment with an identically appearing coil that produced the same sound and was the same weight as the active coil but had negligible magnetic field strength. Scalp sensations in the control subjects were simulated using the sham system described by Borckardt et al. (
46) Before each treatment, study subjects were instructed to insert earplugs. The head of the seated patient was secured in a head coil holder chair and stand assembly (Rogue Research, Montreal). Motor threshold (MT) was ascertained by delivering single pulses to the area of the motor cortex (right side) controlling the contralateral abductor pollicis brevis (APB). Electrical activity in the APB was recorded using surface electrodes. MT was defined as the lowest intensity of stimulation producing motor-evoked potentials of at least 50 µV in five out of 10 trials. MT was determined before the first treatment and then weekly thereafter. The stimulation site for LFR over the DLPFC was F4 of the International 10–20 System for Electrode Placement. Twenty daily (Monday through Friday) rTMS treatment sessions consisting of 1,200 pulses/session at 110% MT were conducted over 4 weeks. Each session included four trains of 300 pulses administered at 1 Hz separated by an intertrain interval of 60 seconds. This parameter set was within established guidelines per the most recently updated TMS safety guidelines (
47). Treatment was delivered by a TMS technician, a coinvestigator (IR), or another physician credentialed to deliver TMS at The Johns Hopkins Hospital and approved by the Johns Hopkins Medicine Institutional Review Board.
Outcome Measures
The primary analysis was comparison of the fitted slopes of the HAM-D scores estimated from all the time points (weeks 4, 8, 12, and 16) from a longitudinal mixed-effects model. In addition, response rates (as defined by a >50% reduction in HAM-D score at week 16) and remission rates (as defined by a HAM-D score ≤7 at week 16) were calculated. Secondary analyses included longitudinal mixed-effects models to compare fitted slopes between study arms on CGI-I, BSSI, comorbid neuropsychiatric symptoms, and cognitive scores. Additionally, exploratory analyses included examination of 1) changes in white matter integrity, as determined by change in FA and trace values, assessed using DTI, at week 16 compared with preintervention values, and 2) changes in polysomnogram results at week 16 compared with preintervention results.
Calculation of Sample Size
The sample size calculation for this study was based on the study conducted by Fann et al. (
48) and our clinical experience. In their open-label study of sertraline for the treatment of TBI depression, Fann et al. found a response rate (≥50% reduction in HAM-D score) of 87% and a remission rate (HAM-D score ≤7 at the end of treatment) of 67%. On the basis of these results, we hypothesized improvement in symptoms of 60%−70% in the experimental group, as measured with HAM-D, and on the basis of our clinical experience, we hypothesized a remission rate of 10% in the control group. We calculated sample sizes using various power levels (0.6, 0.7, 0.8, and 0.9) and experimental remission rates of 0.6 and 0.7. Using power of 0.8 and a remission rate of 0.6, the total sample size was determined to be 34, with N=17 in each arm. For power of 0.9 and a remission rate of 0.6, the total sample size was determined to be 42, with N=21 in each arm. Using power of 0.8 and a remission rate of 0.7, the total sample size was determined to be 26, with N=13 in each arm. For power of 0.9 with a remission rate of 0.7, the total sample size was determined to be 30, with N=15 in each arm. Because this is a pilot study, we chose a sample size (N=30) that was feasible and that would allow us to complete the study in the allocated time. Results from this study will help us to develop future studies with larger sample sizes.
Statistical Analyses
Basic exploratory analyses were conducted first, including checking of distributional assumptions, assessment of relationships among covariates, and missing-value multiple imputations. All study subjects were tracked over the study period in order to capture the number of subjects randomly assigned to each group and the number of participants who dropped out or were lost to follow-up. The two groups (active intervention with rTMS and sham treatment) were compared on demographic and baseline clinical variables. Mixed-effects models were used to evaluate the efficacy of the intervention, with comparison of the slopes in the rTMS (active intervention) and sham treatment (control) groups adjusting for potential confounders. In this model, the coefficient of interest was the interaction between time and treatment assignment, because it represented the difference in rate of change over time in the two groups. To assess the effects of LFR rTMS for treatment of depression and comorbid neuropsychiatric symptoms, effect sizes were calculated by a Hedge’s g-like formula (last visit time × coefficient/pooled standard deviation at baseline), and thus the between-group difference was akin to a fitted difference in mean but without the intercepts. Analyses were conducted using Stata 15.1 (StataCorp, College, Station, Tex.). All statistical tests were judged for significance based on a two-sided p value <0.05.
Results
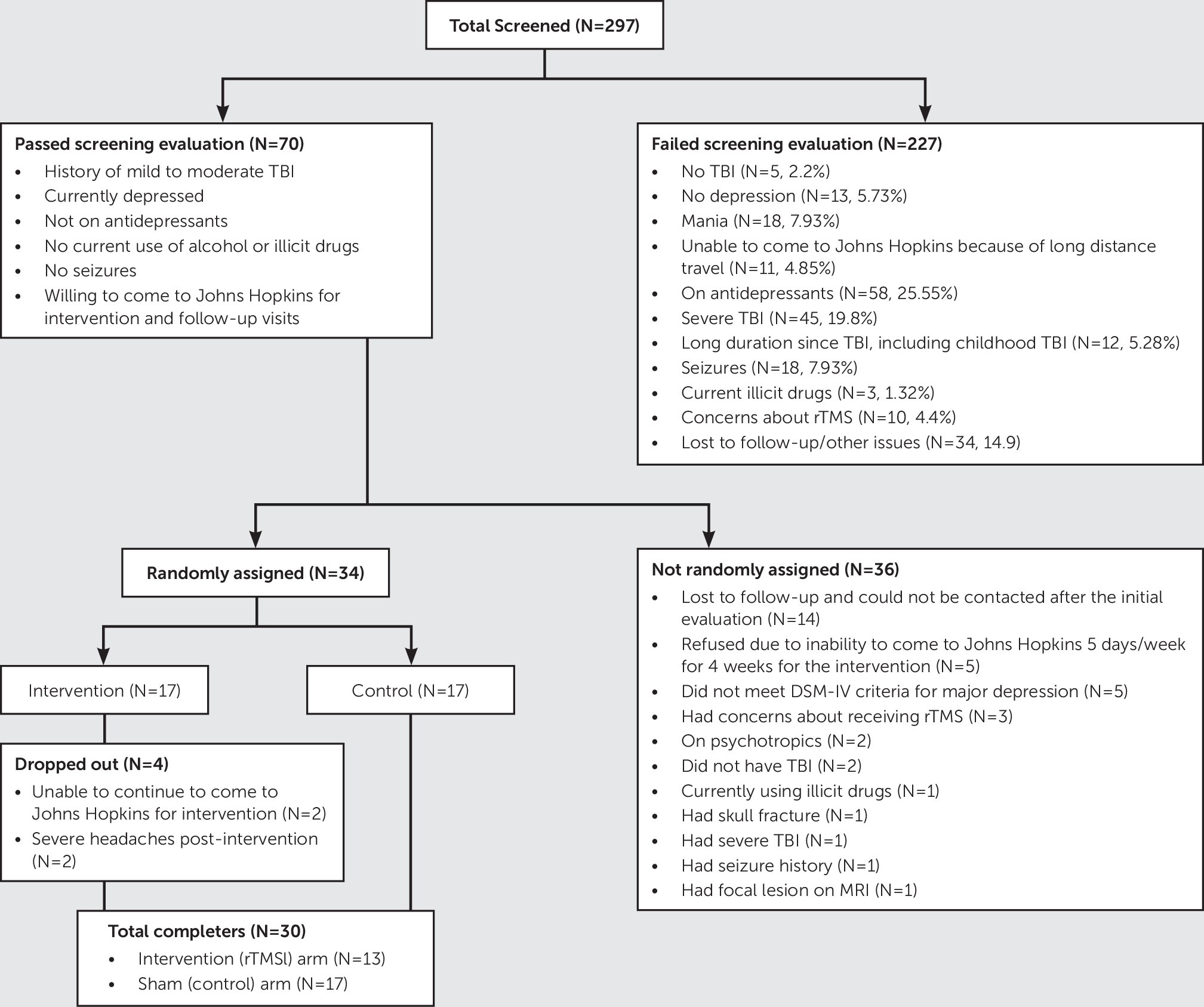
The number of people who were screened, the number of people who were randomly assigned, and the total number of individuals who completed the study and were included in the analysis are shown in
Figure 1. On comparison of participants who were not randomly assigned (N=36) with those who were randomly assigned (N=34), the nonrandomized group had lower mean HAM-D scores (mean, 22.1 [SD=4.8] versus mean, 25.4 [SD=5.3], p=0.029) and positive family history of nonmood disorders (68% in the nonrandomized group versus 41% in the randomized group, p=0.04). There were no other statistically significant differences on demographic or clinical variables. Similarly, on comparison of individuals who dropped out (N=4) with those who completed the study (N=30), study drop outs had higher mean scores on the Modified Overt Aggression Scale (mean, 8.8 [SD=10.8]) versus mean, 2.7 [SD=3.3], p=0.018) and higher percentages of post-TBI comorbid psychiatric disorders, such as posttraumatic stress disorder or generalized anxiety disorder (67% versus 7%, p=0.004), suggesting that they may have been in poorer condition. There were no significant differences on demographic or clinical variables. Because the goal of this study was to have 30 completers, all subsequent results are based on this cohort of 30 who completed the study.
Demographic and Preinjury Clinical Variables
Comparison on demographic and baseline clinical variables between participants in the sham arm and those in the rTMS intervention arm is summarized in
Table 1. There were no differences between the two groups on any of the measures at baseline, except for statistically significant higher fatigue scores in the control group, which was controlled for statistically in the analyses.
Blinding Effectiveness
The effectiveness of the blind was evaluated by asking each participant after the first treatment and again at the end of the 20th treatment his or her opinion on the treatment received. After the first treatment, the majority of participants believed that they had received active treatment (N=12/13 in the rTMS intervention arm and N=14/17 in the sham arm), and after the 20th treatment, seven out of 13 participants in the rTMS intervention arm and 13 out of 17 in the sham arm believed that they had received active treatment.
Safety and Tolerability
Of the 34 study subjects who were randomly assigned, four dropped out of the study. All four who withdrew were in the rTMS treatment arm. One dropped out after nine interventions, because she had difficulty coming to the study site regularly (i.e., she was unable to take time off from work). Another withdrew after five treatments secondary to intervention-associated headaches and having to come to the study site regularly. Another withdrew after just one treatment secondary to severe headaches that lasted for 4 days, and another was unable to keep up with the monthly follow-up appointments after the intervention as a result of relocation out of state to pursue educational opportunities.
Among the 30 completers, both groups had a similar number of side effects (
Table 2). The majority of participants in both arms did not experience side effects. Headache was the most common side effect in both groups, with the majority experiencing them at the start of the intervention and during the first month of the study. Other side effects reported in both groups included worsening mood, dizziness, discomfort at the stimulation site, insomnia, and other general effects (e.g., face tightness, toothache). No one reported syncope, cognitive fogginess, or hearing loss, and no seizures were reported.
Primary Outcome
The effect size of LFR rTMS over the DLPFC on TBI depression was assessed with a longitudinal mixed-effects model on the primary outcome measure (HAM-D), after adjusting for fatigue. The effect size (
Table 3) for this analysis was found to be small (Hedges’ g=0.16). Chi-square tests of independence were calculated comparing the responder results in the two groups. There were no statistically significant differences between the two groups on rates of remission, as defined by a HAM-D score <7 (χ
2=0.007, df=1, p=1) or response, as defined by a 50% reduction in the HAM-D score (χ
2=0.048, df=1, p=0.71). For HAM-D scores at different visit times, the effect size varied widely, favoring rTMS treatment at some time points and sham treatment at others, ranging from –0.06 at 4 weeks (favoring sham), 0.01 at 8 weeks (favoring rTMS treatment), –0.13 (favoring sham) at 12 weeks, and 0.19 (favoring rTMS treatment) at 16 weeks (
Table 4).
Secondary Outcomes
The effect size for comorbid neuropsychiatric symptoms also varied widely, favoring treatment for symptoms of suicide (as assessed with BSSI), posttraumatic stress (as assessed with DTS), excessive daytime sleepiness (as assessed with ESS), overall neuropsychiatric symptoms (as assessed with NBRS), aggression (as assessed with the Modified Overt Aggression Scale), fatigue (as assessed with FSS), satisfaction with life (as assessed with SWLS), and social ties (as assessed with STC), with effect size ranging between 0.06 and 0.38. The effect sizes favored sham treatment for global improvement (as assessed with CGI-I), sleep (as assessed with PSQI), anxiety (as assessed with GAD), and postconcussive symptoms (as assessed with RPQ). The effect size ranged between –0.03 and –0.53 (
Table 5).
Neuropsychological functioning.
Of the 30 completers, 29 underwent cognitive testing pre- and postintervention. One study subject was unable to participate, because he had difficulty speaking and comprehending English. In terms of the effect size for the different cognitive tests, the WCST was found to have a large effect. However, while the number of correct responses (Hedges’ g=1.15) and errors (Hedges’ g=1.03) on the WCST favored the sham treatment, the number of categories completed (Hedges’ g=1.27) favored rTMS treatment. The effect size for the other tests were relatively small, ranging between 0.02 and 0.39. Some favored rTMS treatment (BVMT-immediate recall, Hopkins Verbal Learning Test-immediate recall, Stroop test-inhibiting automated responses), whereas others favored the sham condition (MoCA total score, processing speed as assessed with the Trail-Making Test, Part A, BVMT-delayed recall, Hopkins Verbal Learning Test-delayed recall, and cognitive flexibility as assessed with the Trail-Making Test, Part B) (
Table 6).
White matter integrity.
All 30 study subjects were invited to undergo structural MRI brain scans. Twenty-six underwent preintervention brain MRIs, and four refused to undergo MRI scans secondary to claustrophobia. Nineteen participants underwent postintervention brain MRIs. Four refused postintervention scans secondary to claustrophobia, and seven were not interested in having a second scan. Nineteen study subjects had normal brain scans; one was found to have extensive bilateral subcortical and periventricular white matter small vessel ischemic changes, more than expected for his age; four had nonspecific subcortical white matter foci; and two had asymmetry of their temporal lobes, with the left smaller than the right, but otherwise normal.
For the DTI analysis, the two groups were first compared on seven primary anatomical areas. No statistically significant differences on both FA and trace values pre- and posttreatment were observed between the two groups. By using the results of whole-brain analysis, the two groups were compared on FA and trace values pre- and posttreatment. Effect sizes were calculated for those regions that reached statistical significance on pre-post comparisons on FA (the right fusiform gyrus, right middle temporal gyrus, left fusiform gyrus, left parahippocampal gyrus) and trace values (the right superior frontal gyrus, right middle frontal gyrus, right middle fronto-orbital gyrus, right insula, right fornix, left cingulate gyrus, left superior frontal gyrus, left middle frontal gyrus, left inferior frontal gyrus, and left caudate) The effect size for FA was found to be mostly small (Hedges’ g=0.01–0.19), except in the left fusiform gyrus (Hedges’ g=0.42), which is a medium effect (
Table 7). For trace values, the effect size was found to be moderate and ranged from 0.35 to 0.78 (
Table 8).
Polysomnogram changes.
All study subjects were invited to undergo two overnight sleep studies, with the first night being for adaptation and the second for data collection. Only 10 participants underwent a preintervention sleep study, eight underwent a postintervention study, and seven participated in both pre- and postintervention studies. The primary reasons for nonparticipation in a sleep study were difficulty staying at the Hopkins Sleep Lab for 2 nights and 1 day (secondary to work- or family-related issues) and preferring to start the study intervention as soon as possible without waiting for a sleep study to be scheduled. No differences were observed in the results of the postintervention polysomnograms compared with the preintervention somnograms among the seven study subjects who participated in both.
Discussion
LFR rTMS was found to be safe and well tolerated, but small and variable effect sizes between the active and sham groups were observed. However, because this was a pilot study, conclusions should be drawn cautiously from the results given the small sample size. Results should only be considered preliminary, because there are several limitations to the study.
The small sample size is the main limitation of this study and precludes us from drawing strong conclusions from the findings. The power analysis we used was based on an open-label medication study and clinical experience and not on previously reported effect sizes of rTMS. A meta-analyses of rTMS on idiopathic depression by Slotema et al. (
29) demonstrated that the mean weighted effect size (expressed as Hedges’ g) of rTMS versus sham for depression was 0.55 (p<0.001). By using this effect size of approximately 0.5 to test the hypothesis that rTMS is more effective than sham for the treatment of depression after TBI, with 80% power, at an alpha level of 0.05 (two-tailed), a sample of more than 130 study subjects (i.e., more than 65 study subjects per group) would be necessary to detect differences between the two groups. Given the sample size of the present study (rTMS group, N=13; sham group, N=17), with 80% power at an alpha level of 0.05 (two-tailed), we would need to have an effect size of ≥1.0 to detect a difference between rTMS and sham for depression after TBI. The observed effect size of 0.2 at week 16 suggests that more than 400 study subjects per group would be needed to demonstrate a beneficial effect of LFR rTMS over the DLPFC for TBI depression with 80% power at a two-tailed alpha level of 0.05 (
49).
There are several other limitations to this study. We used a focal double 70-mm air-cooled coil (a figure of eight coil) to stimulate the right DLPFC at a low frequency of 1 Hz. In addition, treatment was at 110% of the motor threshold, which is more cautious than the more typical 120% of motor threshold used with HFL rTMS for idiopathic depression. We specifically chose LFR rTMS because of our concern for seizures, which may have been an overcautious approach given the low rate of posttraumatic seizures among persons with mild to moderate TBI. The frequency, the coil, and the laterality parameters we used may have reduced the potential effect rTMS could have on TBI depression in our study sample. Studies and case reports that have used HFL frontal stimulation for various neuropsychiatric symptoms in persons with TBI have demonstrated its benefits. George et al. (
50) evaluated the effectiveness of high-dose rTMS to reduce suicidal ideation among hospitalized veterans. For both study groups (active rTMS and sham rTMS), scores on the BSSI declined rapidly over 3 days, with more rapid decline on the first day with active rTMS falling short of statistical significance. Leung et al. (
51,
52) observed improvement in the severity, frequency, and duration of headaches in a prospective case series of six veterans with mild TBI and posttraumatic headaches and later in a second study of 29 veterans with chronic mild TBI and posttraumatic headaches.
It is also possible that the focal double 70-mm air-cooled coil we used may not have stimulated deeply enough to reach neurons and white matter tracts most strongly implicated in TBI depression. Deep TMS H-coil technology is a novel development in TMS designed to achieve effective direct stimulation of deep neuronal regions (
53,
54), and this technology, if used, may have had greater effect.
The heterogeneity in the time since injury (from about 3 months to >10 years) may have also confounded these results. The pathophysiology of TBI is complex, with different types of secondary injuries predominating at times during the recovery course. Therefore, the most appropriate postinjury period for a neuromodulatory intervention to produce maximum benefits is unclear. It may be that any signal of improvement with the intervention was therefore lost among the variability of the postinjury time spans.
Time of assessment for response and remission status was 16 weeks after the end of treatment. It is possible that treatment effects, if present, had dissipated by this time after treatment. The length of treatment was 4 weeks. Increased treatment length (6–9 weeks instead of 4 weeks) may have resulted in a larger effect size. Dropouts were only in the experimental group. It is possible that these dropouts may have had more significant depression, and these patients would have had lower sham response rates and thus showed more benefit from the experimental treatment.
Our study had very rigid inclusion and exclusion criteria, which reduces the generalizability of the study to the TBI population at large. In order to keep it a clean study of rTMS efficacy, we excluded people with depression who were on antidepressants. However, this exclusion criterion may have contributed to the smaller and inconsistent effect size. Several study subjects were excluded because of severe TBI and our concern for seizures. While our concern was safety related, people with severe TBI have high rates of depression and likely more damage or dysfunction to the fronto-subcortical circuits; accordingly, they may have responded to this purely biological treatment in a different manner. People with MMSE scores ≤24 were also excluded. We chose to include people with fairly intact cognitive functioning given the complexity of the study (i.e., use of an intervention that has not been well studied in this patient population, long duration of follow-up [5 days/week for 16 weeks, followed by monthly follow-up for 3 months], and extensive pre- and postintervention assessments, including sleep studies and brain scans). However, persons with TBI often have cognitive deficits, and this exclusion criterion may have also compromised the generalizability to the TBI patient population. Additionally, people with frontal lesions were excluded from the study due to concerns that rTMS over the site of the lesion (frontal) would increase the seizure risk. This exclusion criterion may have also affected the generalizability of the study, because frontal lesions are common in TBI.
Despite these limitations, this study has a number of strengths. To our knowledge, this is the first randomized-controlled trial to evaluate the benefit of rTMS in the treatment of TBI depression and its comorbid psychiatric symptoms and as such may be useful in guiding future studies. A major strength of this study was the use of a sham TMS device, which the study subjects could not readily distinguish from the experimental treatment. Additionally, in the design of the study, we considered preinjury factors, postinjury factors, and injury-related factors. Finally, multiple types of outcome measures (well-validated standardized rating scales, brain imaging parameters, polysomnogram results, and neuropsychological testing results) were used to capture the potential impact of the intervention on the treatment of TBI depression.
Conclusions
Results from this study could be used as pilot data for larger studies to determine the benefits of rTMS for the treatment of TBI depression. Larger, more adequately powered multicenter studies need to be undertaken to assess whether LFR rTMS is effective for treatment of TBI depression. Other factors that need to be examined in future studies include pulse frequency (high versus low), lateralization (left versus right), focality (figure of eight coil versus H-coil), number of sessions (more sessions versus fewer sessions), severity of injury (mild versus moderate to severe), time since injury (acute versus chronic), and comorbid medical and psychiatric issues.
In future studies, there could be consideration of factors that facilitate more personalized treatment for an individual patient, such as administering LFR rTMS for patients who have experienced seizures secondary to their TBI but administering HFL rTMS in milder TBI cases where seizures are less of a risk factor. Future studies may also focus on H-coil (or deep) rTMS for patients with TBI, which has a deeper and wider distribution of magnetic field than standard coils and thus potentially provide greater benefit.