Transient global amnesia (TGA) is an uncommon amnestic syndrome characterized by a combined anterograde and retrograde amnesia of sudden onset.
1–3 Though generally self-limited with rare recurrences, this striking presentation has spurred efforts to link TGA to more consequential and treatable conditions. Previous studies on TGA have been inconsistent regarding sex prevalence.
4–6 The average age of onset has ranged between 50–80 years, with an incidence of approximately 5–11 per 100,000 per year.
7,8Despite the general acceptance of TGA as a clinical entity, its etiology has yet to be determined.
9 Past studies have attempted to correlate TGA to vascular risk factors, migraine, and epilepsy, with variable results. Because of the implicated loss of declarative memory, ischemia in bilateral hippocampi has been suggested as a possible cause. High-resolution brain MRI of TGA patients suggests diffusion restriction in the hippocampi.
10–13 More recent studies have focused on the discovery that a large percentage of TGA patients have jugular venous insufficiency.
14,15 Migraines and epilepsy have also been implicated as potential correlates of TGA.
16 Numerous associations with other possible triggers have been reported
2 yet, none of these correlations have been validated in a nationwide database.
In an effort to better characterize TGA, we have examined the Nationwide Inpatient Sample, which is uniquely suited to evaluate the incidence of rare diseases, such as TGA.
Methods
Data were obtained from the Nationwide Inpatient Sample (NIS) for the years 1999–2008. The NIS is a database maintained as a part of the Healthcare Cost and Utilization Project by the Agency for Healthcare Research and Quality. It represents 20% of U.S. community hospitals, comprising 5–8 million inpatient records per year. The data were collected in a manner whereby we could perform weighted analysis representing the total population of the United States. Information collected by the NIS includes basic demographic data, ICD-9 diagnoses, procedural coding, financial data, and discharge data, including the length of stay and total cost of hospitalization. The sampling strategy selects hospitals nationwide from the State Inpatient Database according to defined strata based on ownership, bed size, teaching status, urban or rural location, and region. All discharges from sampled hospitals for the calendar year are then selected for inclusion into NIS. To allow extrapolation for national estimates, both hospital and discharge weights are provided. Detailed information on the design of the NIS is available online (
http://www.hcup-us.ahrq.gov). The unit of analysis was the discharge or encounter rather than the individual. We assumed that each discharge or encounter appeared only once per patient. A unique hospital identifier allows linkage of discharge data to an NIS data set with hospital characteristics.
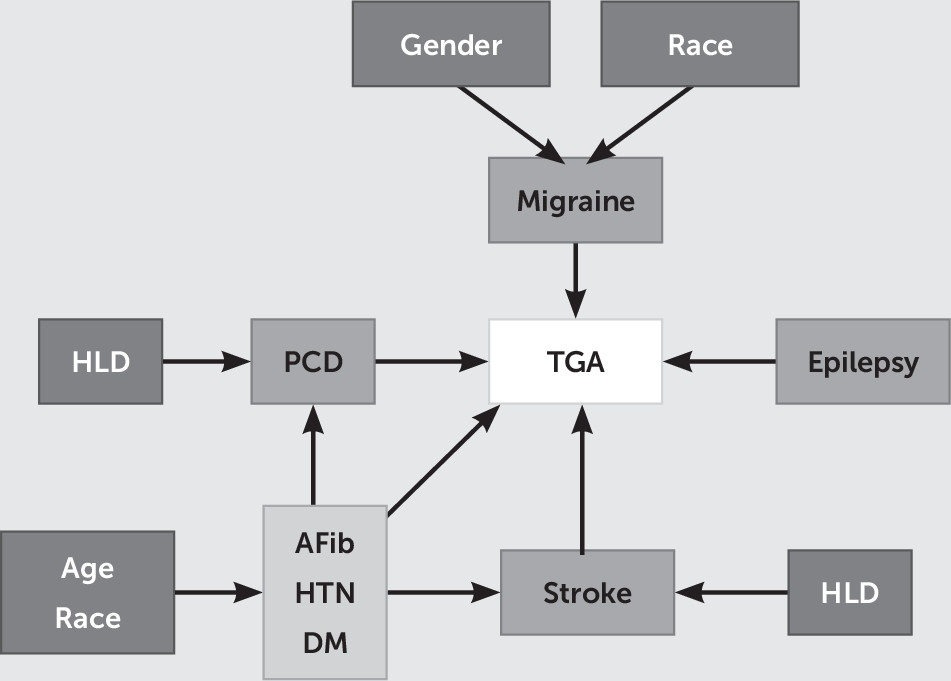
To analyze TGA hospitalizations, we identified all hospital discharges for which an ICD-9-CM code of 437.7 (transient global amnesia) was listed. Searching the literature, we were able to identify eight comorbidities previously associated with TGA: epilepsy (ICD-9 code 345), migraine headache (ICD-9 code 346), precerebral disease (atherosclerosis) (CCS code 110), stroke (CCS code 109), atrial fibrillation (CCS code 106), hypertension (CCS code 98), hyperlipidemia (CCS code 53), and diabetes mellitus (CCS codes 49, 50). Missing data were not included in the analyses. The variables included were a binary outcome (TGA), four main binary predictors (precerebral disease, migraine headache, epilepsy, and stroke), and three potential binary confounders (atrial fibrillation, hypertension, and diabetes mellitus) as well as other secondary variables (sex [binary], race [nominal], age [continuous], hyperlipidemia [binary], and hospital classification [region, location, and teaching status; binary]). From the original hospital discharge records (from 1999 to 2008), we were able to identify 58,191,569 patients without TGA and 9,723 patients with TGA. A causal diagram that graphically represents assumed associations and compounding relationships among selected variables and TGA is shown in
Figure 1.
Statistical Analysis
Using chi-square tests, we explored the differences between TGA and non-TGA hospitalizations. Differences in the distribution of the study variables were tested by Chi-square test for independence. Independent predictors and covariates examined included: a) socio-demographic characteristics b) clinical factors and c) selected two-way interactions. Multiple logistic regression analyses were performed to explore the relationship between TGA status and selected predictors. Since the NIS database uses a stratified, single-stage random cluster sampling methodology, we used a survey logistic regression to account for discharge weights and effects of stratification and clustering. We combined NIS data from 1999 to 2008 and ran the final model with all demographic variables and comorbidities. Adjustments in the multivariable models were made for socio-demographic factors, including age, sex, and race, clinical comorbidities and hospital regions. Age in years was centered at 65, and a squared term was added to the model to determine if TGA has a quadratic relationship with age. We ran two models, one with race-comorbidity interaction and the other without the interaction, with the aim being to ascertain the average effect for race.
Only patient records with no missing data for selected model variables were included for analyses.
NIS sampling and design parameters were employed during this analysis. Demographic statistics were processed with SPSS, version 20, with the complex samples module. Logistic regressions were conducted with Statistical Analysis System, 9.3. This study was deemed exempt by the institutional review board at Loma Linda University.
Results
From the original hospital discharge records for all potentially eligible patients, between 1999 and 2008, the study included 58,191,569 unweighted patients without TGA, which was equivalent to 283,228,304 weighted patients without TGA, and 9,723 unweighted patients with TGA, equivalent to 47,432 weighted patients with TGA (
Table 1). Any hospital discharge for which any chosen variables were missing was excluded from the analyses.
The 47,432 patients in the TGA group differed from the patients in the non-TGA group in age (TGA group: mean age, 64.93 years [SD=0.18], non-TGA group: mean age, 47.74 years [SD=0.25]). Most patients in the TGA group were ≥65 years old (55.04%). Overall, Caucasians outnumbered other ethnicities in both groups, with more Caucasians in the TGA group (86.28% versus 67.86%). Other racial and ethnic populations were represented at noticeably lower proportions in both groups (TGA group: African American, 4.43%, Hispanic, 5.30%, and Asian/Other, 4.0%; non-TGA group: African American, 13.6%, Hispanic, 12.42%, and Asian/Other, 6.12%). There was a higher proportion of women in both groups, with more women in the non-TGA group (51.99% versus 58.8%).
Total in-hospital charges were less for the TGA group compared with the non-TGA group ($14,242 versus $21,319), but the cost trend increased over the 10-year period. Overall, TGA hospitalization was associated with a reduced number of procedures (TGA group: mean, 0.69 [SD=0.036]; non-TGA group: mean, 2 [SD=0.016]), yet patients in the TGA group were seven times more likely to have brain MRI (7.66% versus 0.44%) and head CT scans (7.31% versus 0.87%). Similarly, more lumbar punctures were performed for patients with TGA (3.23% versus 0.91%). Length of hospital stay was shorter for patients in the TGA group, with a mean of 2.49 days (SD=0.036), compared with 4.72 days (SD=0.25) for patients without TGA. More patients with TGA were evaluated at urban hospital settings (urban setting, 90.16%; rural setting, 9.84%) and in the Southern region (37.45%).
We were able to identify differences in comorbidities using Charlson’s comorbidity index.
17 Hypertension was twice as prevalent among patients with TGA (51.25% versus 27.62%). Valvular disease was detected more frequently in the TGA group (8.26% versus 5.0%) as well as anxiety (5.98% versus 2.99%) and depression (8.88% versus 6.81%). Overall, TGA hospitalization was associated with lower mortality (0.1% versus 2.24%), routine discharges from the hospital (91.46% versus 74.49%), and less frequent discharge to a facility or home health care (6.82% versus 20.06%).
Significant differences were observed in the distribution of medical comorbidities. Of particular importance was the high prevalence of migraine headache, hypertension, precerebral disease, and hyperlipidemia among patients with TGA, when compared with non-TGA patients. Migraine was almost five times more prevalent in the TGA group compared with the non-TGA group (4.94% versus 0.76%), and hypertension was twice as high (51.25% versus 27.62%), precerebral disease almost four times as high (3.94% versus 1.0%), and hyperlipidemia nearly three times more prevalent (33.66% versus 12.55%). However, this pattern was not seen for epilepsy (0.79% versus 0.64%) (
Tables 2 and
3).
Using survey logistic analysis, we found that the odds of having TGA was higher for men (odds ratio=1.13, 95% confidence interval [CI]=1.08–1.18) (
Table 4). The squared-age term was significant, and thus it was retained in the model. In the adjusted regression model, patients with migraine headache had a sixfold greater odds of TGA compared with patients in the non-TGA group (odds ratio=5.98, 95% CI=5.42–6.60), and migraine headache was followed by precerebral disease (odds ratio=1.92, 95% CI=1.71–2.14), hyperlipidemia (odds ratio=1.88, 95% CI=1.79–1.97), and hypertension (odds ratio=1.35, 95% CI=1.29–1.41). The adjusted odds ratio for epilepsy was 1.16 but was not statically significant (95% CI=0.92–1.45.). TGA was not associated with stroke (odds ratio=1.00, 95% CI=0.89–1.14). Patients with atrial fibrillation and diabetes had lower odds of TGA (atrial fibrillation: odds ratio=0.69, 95% CI=0.64–0.73; diabetes: odds ratio=0.36, 95% CI=0.33–0.39).
Compared with Caucasians, the odds of having TGA was lower among African Americans (odds ratio=0.36, 95% CI=0.23–1.34) and Hispanics (odds ratio=0.69, 95% CI=0.09–0.56) as well as among individuals classified as Asians/Other (odds=0.82, 95% CI=0.12–1.10) (
Table 4).
African Americans with precerebral disease had almost a fourfold increased odds of having TGA (odds ratio=3.72, 95% CI=2.15–6.42), and Hispanics with precerebral disease had almost three times the odds (odds ratio=2.72, 95% CI=1.40–5.28), while Caucasians with the disease had almost twice the odds (odds ratio=1.92, 95% CI=1.71–2.14). For patients with hyperlipidemia, the odds of TGA was almost twice as high among Caucasians (odds ratio=1.88, 95% CI=1.79–1.97) and Hispanics (odds ratio=2.11, 95% CI=1.70–2.61), but a statistically significant association was not seen among African Americans (odds ratio=1.24, 95% CI=0.96–1.58). Diabetes seemed to be protective for TGA in all races but more for Hispanics, Asians/Other, and Caucasians than for African Americans (Caucasians, odds ratio=0.36, 95% CI=0.33–0.39; African Americans, odds ratio=0.56, 95% CI=0.43–0.71; Hispanics, odds ratio=0.31, 95% CI=0.24–0.340; Asians/Other, odds ratio=0.36, 95%=CI 0.27–0.47) (
Table 5).
Discussion
Consistent with previous data, the average age of patients with TGA was approximately 65 years,
4 and our adjusted regression model revealed that men had modestly higher odds of having TGA. Our most significant finding was that those with a diagnosis of migraine had six time greater odds of having TGA. Migraine and TGA, as historically noted, share a number of characteristics, including paroxysmal presentation and associated triggers. It has been proposed that cortical depression in the hippocampal region is the common denominator in both disease processes.
18–21 Several studies have gone as far as to refer to TGA as a migrainous aura.
20,22 Despite the proposed shared pathophysiologic mechanisms, there are several unavoidable differences, such as TGA being self-limiting and most often affecting elderly men.
Because there was significant interaction between comorbidities and race, the effect of comorbidities was not consistent when considering racial differences. We found that for African Americans, diagnosis of precerebral disease increased the odds of having TGA by 3.7 times, and the odds was increased by 2.7 times for Hispanics and almost two times for Caucasians. Similarly, the odds of hyperlipidemia were elevated among Caucasians, Hispanics, and Asians but not African Americans. Contrary to previous studies, we did not find a statistically significant association between epilepsy and TGA. The low recurrence and self-limiting nature of TGA make it highly unlikely to be related to epilepsy. TGA patients typically have normal EEG recordings during and after a genuine episode of TGA.
23–25 At times, the postictal amnesia associated with partial complex seizures may last longer than usual. However, such events are uncommon and are associated with ictal manifestations, and therefore, must be considered as a distinct diagnosis of epilepsy, rather than TGA. The finding of elevated odds of TGA with hyperlipidemia, precerebral disease, and hypertension as opposed to Seizures speaks to a more vascular possible etiology to TGA than an electrophysiological one.
Conflicting reports exist with regards to thromboembolic processes and their correlation with TGA symptomatology. Multiple neuroimaging studies have noted the presence of ischemic changes in the hippocampi during different stages of TGA.
26 In a case-control study, Lauria et al.
5 describe decreased frequency of patent foramen ovale in TGA patients compared with TIA patients, and concluded that cryptogenic thromboembolic processes were unlikely to be responsible for TGA. Similar to previous studies, we did not find a correlation between TGA and stroke. This is an important finding, as it suggests that patients with a clear diagnosis of TGA do not require inpatient admission and extensive diagnostic workup.
We found that the cost of TGA hospitalization has increased disproportionately over the last ten years. One could posit that increase in the use of sophisticated and costly neuroimaging modalities is largely responsible for the climbing costs of TGA care, and suggest that a better understanding of the disease process and definitive diagnostic criteria might obviate the need for such extensive workup.
We also found geographic differences in TGA diagnosis. Patients in hospitals located in the Southern region were more likely to be diagnosed with TGA, with relatively fewer diagnoses in the Midwestern and Northeastern regions, and least frequently in Western region of the country. There is indication in the Healthcare Cost and Utilization Project database that the Southern territory has a relatively larger number of hospitals, and more academic institutions per capita than the West. The distribution of neurology specialists and neurology training programs is similarly skewed. Large centers in the East Coast are more likely to be located in proximity to larger population. Due to stratified and single-stage cluster sampling of this database, our findings can be generalized for the greater U.S. population.
Limitations
Although the numerical power of our study is unprecedented, it is likely that this number is still an underestimation of the true prevalence of the disease, due to a variety of factors, including misdiagnosis, noninpatient evaluations, and regional differences. Because the diagnosis of TGA is self-limited and typically lasts only 4–6 hours, we must consider the possibility that patients with TGA may never present to the hospital, let alone be admitted.
It is very difficult to account for the inverse relationship between TGA and such comorbidities as atrial fibrillation and diabetes, other than the possibility of physician bias, where those physicians, in an emergency room setting, are more likely to diagnose patients with symptoms consistent for TGA as having stroke, or transient ischemic attach if they also have a comorbidity of diabetes or atrial fibrillation. This also may speak to the limitations of this database for diagnosis of TGA, but the fact that this category of bias would bias toward the null, would only strengthen the possible degree of relationships that we were able to observe.
The NIS, by virtue of its focus on the inpatient population, is unable to account for the possibility of diagnosis and conservative management in the outpatient clinic and emergency room settings. Additionally, the inherent weakness of NIS is that it relies upon unverified physician diagnosis. We also acknowledge that diagnostic criteria have varied in the past and are not consistently implemented. Despite these limitations, we believe that our results provide great insight into this enigmatic disease as well as direction for future research.