The overwhelming focus on memory following TBI has been on anterograde amnesia, and there has been very little research on retrograde amnesia.
2 This is concerning given that in the handful of studies that have specifically examined retrograde autobiographical memory following TBI, there appears to be a deficit across all lifetime periods tested, at least in severe cases.
3–7 Autobiographical memory is thought to serve several functions, including fostering a sense of continuity in personal identity across time,
8 enabling the application of previously acquired knowledge and experiences to the present,
9 and contributing to social interactions.
10 Autobiographical memory function, therefore, has important implications for psychosocial rehabilitation and reintegration into the community.
Community studies on retrograde autobiographical memory following severe TBI, conducted at variable stages postinjury, suggest that memories are less detailed than among healthy controls (HCs).
3–7,11 Episodic memories, specific to a particular place and time,
12 appear to be particularly affected. Some studies suggest that individuals with TBI also perform more poorly than controls at more semantic levels of retrieval (e.g., lifetime periods and general events that represent a group of related episodes, as opposed to a specific day),
4,13 although this aspect of autobiographical memory has been less extensively investigated. It remains unknown how factual information about the self, also known as personal semantic memory,
14 is affected and to what extent either type of memory difficulty exists among individuals with moderate-severe TBI recruited from consecutive hospital admissions. Esopenko and Levine
5 recently found deficits in episodic autobiographical memory in severe but not mild and moderate cases at one year postinjury, which suggests that there may be different patterns of change over time in different injury severity groups.
It is of interest to look not only at the type and degree of memory deficits but also at the pattern of these deficits across lifetime periods. Take a pure medial temporal lobe (MTL) amnestic syndrome as a quintessential example; when damage is restricted to the hippocampus and surrounding regions of the MTL, there is not only a profound impairment in declarative memory but also a temporal gradient in retrograde memory performance, whereby recent memories are lost and remote memories are spared.
15 When damage is more widespread, or when both fontal and temporal regions are implicated, it is not uncommon for flatter gradients to be observed.
16 Given the frequency of both frontotemporal and distributed injury in TBI, it is relatively unsurprising that the deficit in retrograde autobiographical memory appears to extend across all lifetime periods tested. Memory theories do diverge, however, with respect to the influence of memory age on the storage of episodic and semantic memories. On the one hand, standard consolidation theory would suggest that independence from the hippocampus is governed by the age of the memory.
17 On the other hand, multiple trace theory contends that memories that are episodic in nature always require the hippocampus for retrieval.
18 The type of memory being assessed, therefore, appears to be important when one considers the relationship between anterograde and retrograde amnesia.
Although anterograde and retrograde amnesia co-occur in many amnestic syndromes,
19 each can also occur in isolation. In syndromes such as temporal lobe epilepsy
20 and among MTL amnestic patients,
21 a stronger correlation with anterograde memory function is generally found for more recent autobiographical lifetime periods than for more remote lifetime periods. This is thought to reflect the involvement of the hippocampus in recent but not remote memory retrieval.
21,22 In TBI, where damage often extends outside of the medial temporal lobes, some aspects of new learning have been associated with the more episodic aspects of autobiographical memory retrieval,
4 but this analysis did not distinguish between recent and remote events. Piolino and colleagues
7 only found an association between recent autobiographical memory performance and new learning when a postinjury period was included in their analysis, which was confounded by anterograde memory functioning. When assessing only lifetime periods prior to injury, research on TBI has the advantage of a clear date of onset of both anterograde and retrograde memory problems.
The aim of our study was to address the above gaps in the literature by assessing the prevalence of retrograde episodic and personal semantic autobiographical memory deficits and their relationship with anterograde memory function. We achieved this by assessing patients soon after emergence from posttraumatic amnesia (PTA) and again 6 months later relative to an HC group.
We hypothesized that individuals with TBI would show greater overall impairment in episodic than in semantic retrograde autobiographical memory relative to HC participants. We predicted that individuals with less-severe injuries would display greater improvement in autobiographical memory from the initial to the follow-up time point compared with those with more severe injuries. In addition, we hypothesized that anterograde memory function would be more closely associated with autobiographical memory performance for more recent, as opposed to remote, lifetime periods and that better autobiographical memory performance would be associated with better community integration at the 6-month follow-up.
Results
The sample of 54 participants was predominantly male (75.9%), with a mean age at injury of 38.56 years (SD=17.39; range: 18–81). The mean PTA duration was 22.06 days (SD=13.92; range: 7–64), and the mean lowest preintubation score on the Glasgow Coma Scale within the first 24 hours, obtained from ambulance notes and medical file, was 8.70 (SD=4.45; range: 3–15). All participants remained in PTA for at least 7 days postinjury and were therefore classified as having moderate to severe TBI.
24 The mean education was 12.24 years (SD=2.17; range: 8–18). The cause of injury was motor vehicle accident in 40.7% of cases, pedestrian accident in 18.5%, motorcycle accident in 16.7%, fall in 11.1%, bicycle accident in 7.4%, and horseback riding accident in 5.6%. Abnormal pathology on initial computerized tomography scan was noted in 81.5% of cases, with subarachnoid hemorrhage noted in 65.9%, subdural in 34.1%, extradural in 13.6%, and intracerebral in 54.5% of these cases. Focal cortical contusions were found in 59.1% of cases.
The HC group (N=30) was 70% male, with a mean age of 40.23 years (SD=19.47; range: 18–81). The mean education was 12.13 years (SD=1.70; range: 8–17). The HC and TBI groups did not differ significantly in gender (χ2=0.35, df=1, p=0.366), age (t=–0.41, df=82, p=0.686), or education (t=0.23, df=82, p=0.816).
Follow-up data were missing for 13 TBI case subjects (eight were uncontactable or had scheduling difficulties with multiple attempts, two were overseas, and three declined) and for three HC participants (one declined, and two were overseas or in the hospital). The TBI participants lost to follow-up (N=13) did not differ significantly from the remaining case subjects (N=41) in age (t=–1.47, df=52, p=0.149), years of education (t=0.56, df=52, p=0.575), Glasgow Coma Scale score (t=1.98, df=51, p=0.053), PTA duration (t=–0.68, df=52, p=0.502), or overall PS Schedule memory score on the initial interview (t=1.23, df=52, p=0.223). However, the group lost to follow-up did score significantly higher on the AI Schedule (lost to follow-up, mean=22.19 [SD=2.81]; follow-up group, mean=18.87 [SD=6.32]; t=2.65, df=45.01, p=0.011) when we corrected for unequal variances. Descriptive statistics for each group at initial and follow-up time points are displayed in
Table 2. Preliminary analyses revealed a moderate negative correlation between TBI participants’ average score on the recent life section of the PS Schedule (N=41; r=–0.35, p=0.025) and AI Schedule (N=41, r=–0.38, p=0.016) and overall PTA duration.
The initial interview was conducted an average of 7.43 days (range: 1–32 days) following emergence from PTA. Follow-up interviews were conducted, on average, 6 months later (mean=179.71, range: 153–236 days) for TBI participants and 185.70 days later (range: 145–246) for HC participants. Timing of follow-up did not differ significantly between the TBI and HC groups (t=–1.22, df=66, p=0.227).
Degree of Impairment for AI and PS Schedules
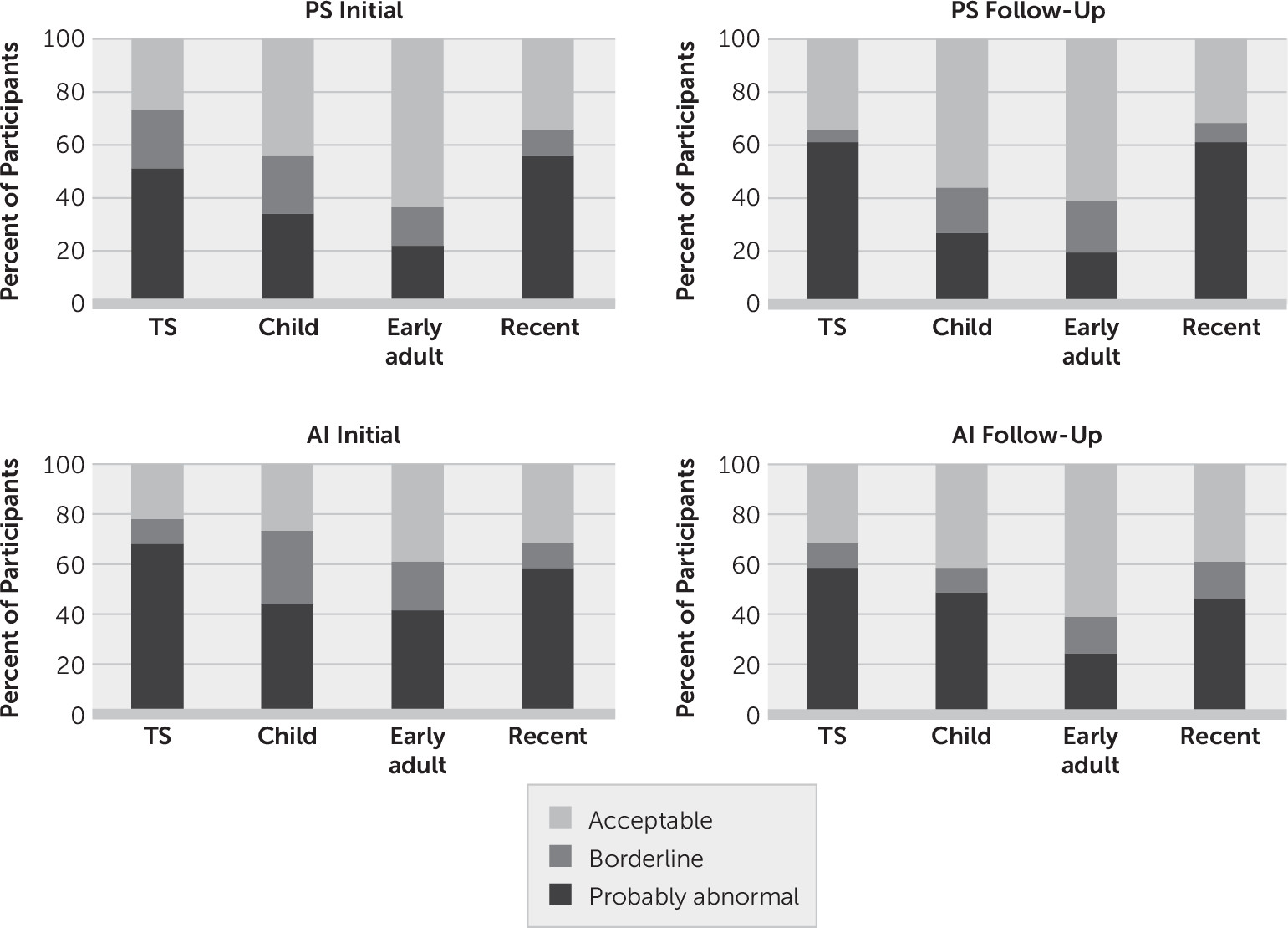
The percentage of participants falling into each impairment category, according to the AMI manual for the PS and AI Schedules, is presented graphically in
Figure 1. The z-score for the total score in the TBI group (calculated relative to the HC group) did not differ significantly between the PS and AI Schedules either initially (t=1.10, df=53, p=0.275) or at follow-up (t=–0.71, df=40, p=0.483). For both schedules, at the initial and follow-up interviews, recent life had the lowest percentage of participants within the acceptable range, followed by childhood and early adulthood, respectively.
Pattern of AMI Performance Over Time According to Injury Severity
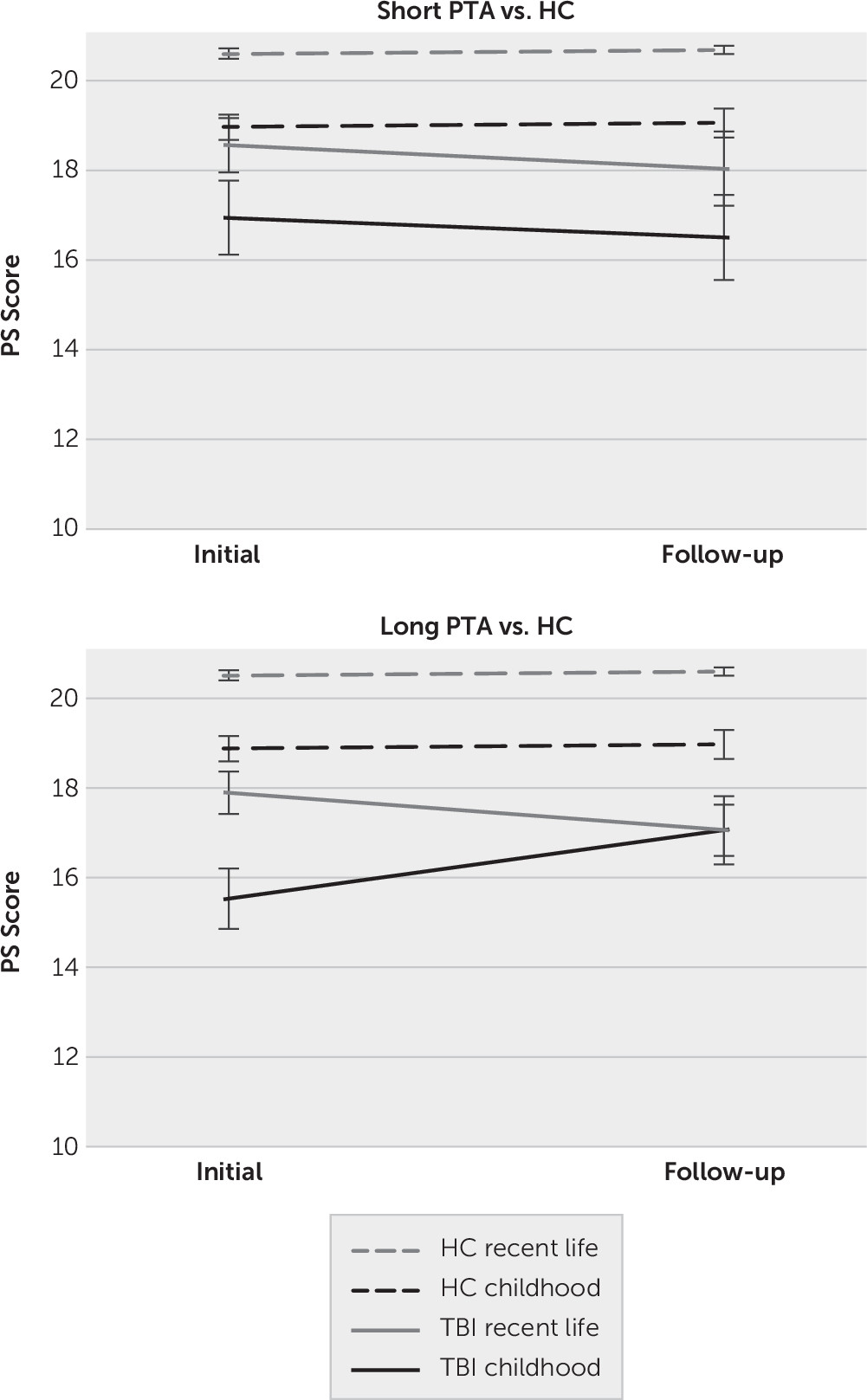
For the PS Schedule, results of the mixed repeated measures ANOVA comparing scores in the HC and TBI groups across time (initial and follow-up) revealed a main effect of group (F=17.54, df=2, 65, p<0.001, ηp2=0.35). Pairwise comparisons indicated that the HC group (mean=19.82 [SE=0.35]) performed significantly better than the long (mean=16.97 [SE=0.37]) and short (mean=17.51 [SE=0.46]) PTA groups (p<0.001 in both instances), although the two TBI groups did not differ significantly from each other. The main effect of lifetime was also significant, whereby scores for recent life (mean= 18.83 [SE=0.25]) were significantly higher than childhood (mean=17.37 [SE=0.32]; F=16.09, df=1, 65, p<0.001, ηp2=0.20). There was no statistically significant lifetime-by-group interaction, nor was there a main effect of time or a time-by-group interaction. However, there was a significant time-by-lifetime interaction (F=4.90, df=1, 65, p=0.030, ηp2=0.07) and time-by-lifetime-by-group’ interaction (F=4.86, df=1, 65, p=0.011, ηp2=0.13).
To unpack this three-way interaction, simple effects analyses were performed to compare each TBI group to the HC group separately (see
Figure 2). This revealed that although the time-by-lifetime-by-group interaction was significant when comparing the long PTA group to the HCs (F=8.32, df=1, 50, p=0.006, η
p2=0.14), this interaction was not significant when comparing the short PTA group to the HC group (F=0.02, df=1, 41, p=0.887, η
p2<0.001). Examining the time-by-group interaction separately within each lifetime period in the long PTA group revealed that the time-by-group interaction was significant for childhood (F=8.13, df=1, 50, p=0.006, η
p2=0.14) but not recent life (F=1.69, df=1, 50, p=0.199, η
p2=0.03). The significant interaction for childhood appeared to be driven by greater improvement in the TBI group from the initial (mean=15.62 [SE=0.51]) to the follow-up (mean=17.14 [SE=0.47]) interview than the improvement in the HC group from initial (mean=18.96 [SE=0.49]) to the follow-up (mean=19.06 [SE=0.45]).
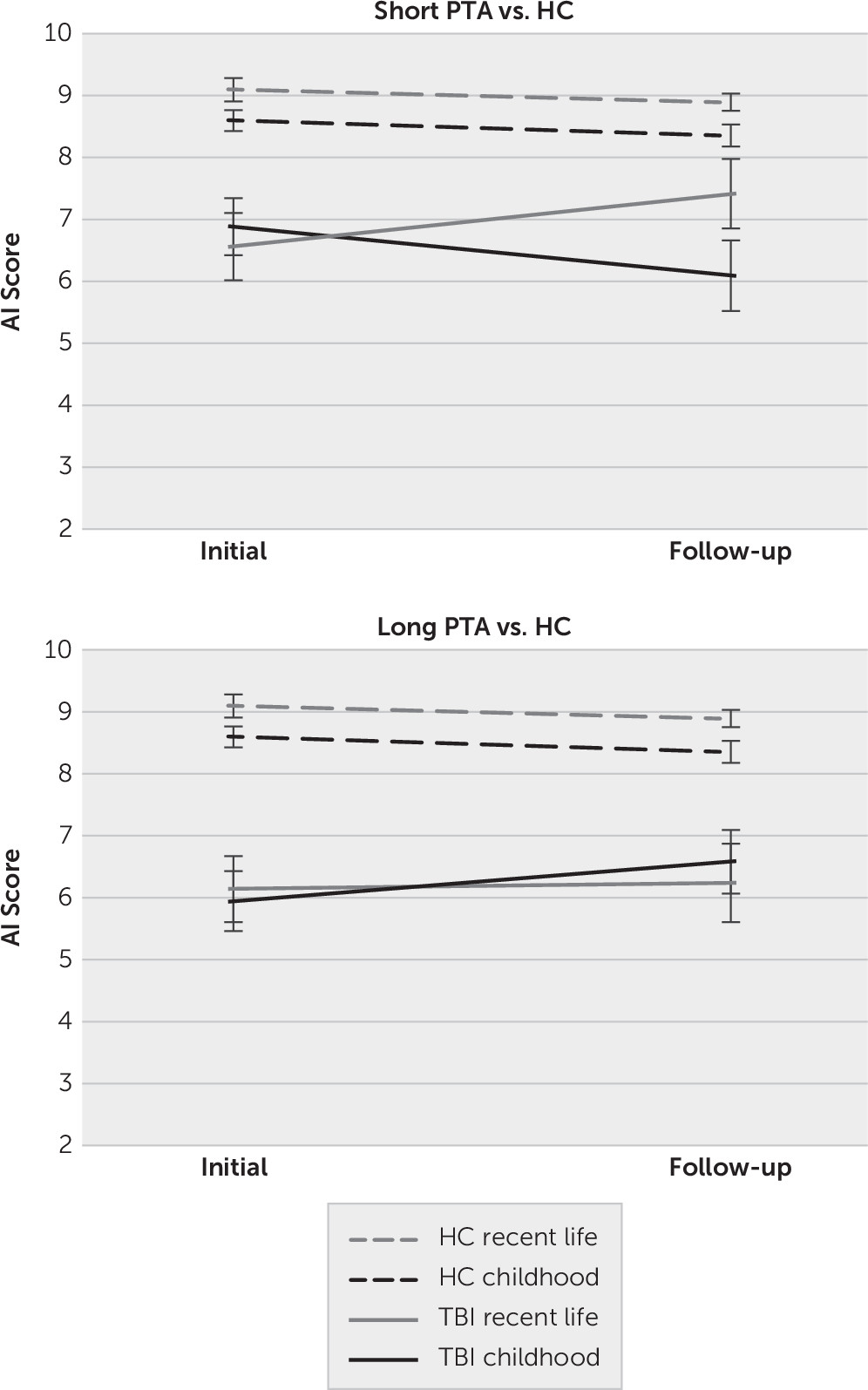
The same analyses were also applied to the AI Schedule. The results were the same using the four or five-point Likert scale scoring method, so only the five-point Likert scale is reported. For the mixed-repeated measures ANOVA comparing scores in the HC and TBI groups across time, there was again a main effect of group (F=17.78, df=2, 65, p<0.001, η
p2=0.35). Similar to the above results for the PS Schedule, pairwise comparisons comparing groups on AI indicated that the HC group (mean=8.73 [SE=0.31]) performed significantly better than the long (mean=6.23 [SE=0.32]) and short (mean=6.73 [SE=0.40]) PTA groups (p<0.001 and p=0.001, respectively), although the two TBI groups did not differ significantly from each other. The main effect of lifetime did not reach statistical significance (F=2.94, df=1, 65, p=0.091, η
p2=0.04). The only interaction effect that approached significance was the time-by-lifetime-by-group interaction (F=2.89, df=2, 65, p=0.063, η
p2=0.08). Visual inspection of the graphs in
Figure 3 suggest that this trajectory toward an interaction was driven by the short PTA group whereby there seemed to be some improvement in recent life scores in the TBI group from the initial (mean= 6.56 [SE=0.50]) to the follow-up (mean=7.41 [SE=0.56]) time point relative to the HC group from the initial (mean=9.09 [SE=0.39]) to the follow-up (mean=8.89 [SE=0.44]) interview. No further statistical analyses were conducted given that the interaction did not reach statistical significance.
Relationship Between Anterograde and Retrograde Memory Performance in the TBI Group
Table 3 shows the Spearman correlations between AMI measures and the RAVLT. Intercorrelations between AMI measures within each group are presented in
Tables 4 and
5. Total learning was significantly positively correlated with recent life scores on the PS Schedule at the initial and follow-up interviews, with moderate effect size (R
2=0.16 and 0.23, respectively). There was also a significant positive correlation between total learning and the recent life section of the AI Schedule at the initial interview (R
2=0.23), but there was no significant correlation between these two variables at follow-up (R
2=0.06). There was also a moderate positive association between total learning and early adulthood scores for the AI Schedule at the initial (R
2=0.12) and follow-up interview (R
2=0.21). However, both of these associations failed to reach statistical significance at the more conservative alpha level (0.01).
The only significant correlation between delayed recall and AMI scores was for the recent life section of the PS Schedule at the initial interview (R2=0.14). The correlation with the recent life section of the AI Schedule at the initial interview was also moderate but fell short of statistical significance (R2=0.11).
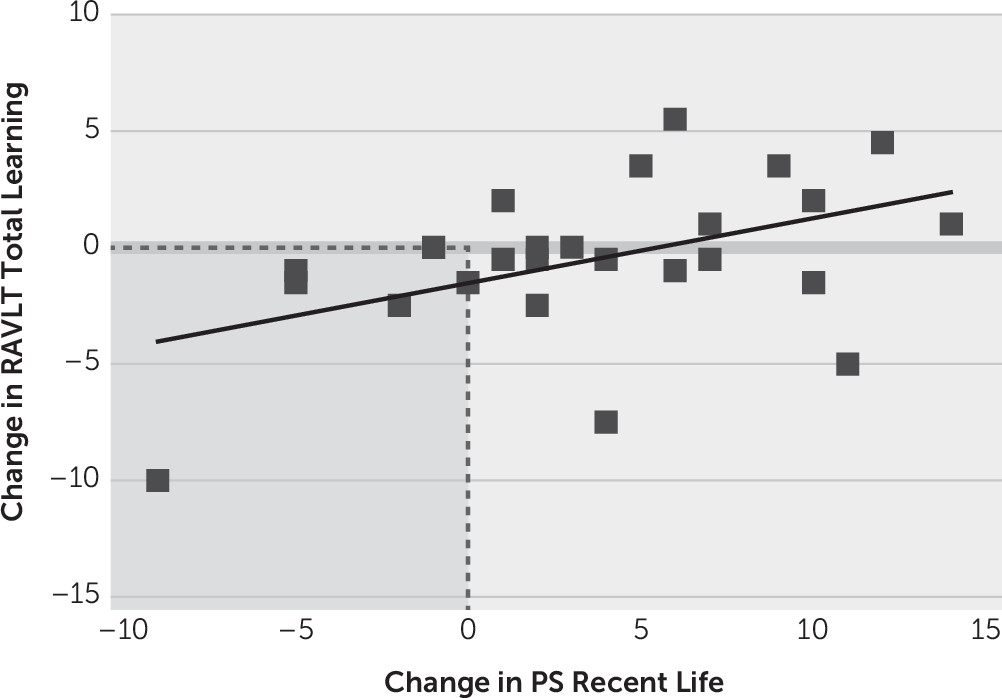
To further explore the association between scores for recent life and RAVLT total learning, we calculated change scores for both measures. These variables were normally distributed, and thus we used Pearson correlations. As depicted in
Figure 4, for the PS Schedule, change in recent life score was moderately positively correlated with change in total learning (N=26, r=0.46, p=0.017, R
2=0.21). There was no significant association between change in recent life score for the AI Schedule and change in total learning (N=26, r=0.04, p=0.850, R
2=0.002).
Association Between AMI Performance and Community Integration
There was a moderate positive correlation between the recent life section of the AI Schedule at the initial interview and scores on the CIQ at follow-up (r=0.36, p=0.016, R2=0.13). However, this correlation did not reach statistical significance when a more conservative alpha level was used (0.01). This trajectory was also observed at the follow-up interview (r=0.30, p=0.067, R2=0.09), although it was further from significance. No other scores at the initial or follow-up interviews correlated significantly with the CIQ.
Discussion
A deficit in retrograde autobiographical memory performance among individuals with TBI has been found in a handful of studies. These studies, however, have typically been cross-sectional in design and conducted with community samples of individuals with chronic and severe TBI. The purpose of the present study was to examine the prevalence of autobiographical memory deficits, as well as their association with anterograde memory function and community integration, among patients who were admitted to a rehabilitation hospital and were still within approximately 1 month of emergence from PTA. The results demonstrated not only that autobiographical memory deficits are common following moderate-severe TBI, but also that both personal semantic and episodic autobiographical memory deficits persist until at least 6 months following PTA emergence.
It was hypothesized that individuals with TBI would be more likely to show episodic than personal semantic autobiographical memory deficits. In the current study, however, there was no significant difference between the TBI participants’ overall degree of impairment in episodic autobiographical incident memory or personal semantic memory relative to the HC group at the first or second interview. For both schedules, a pattern of impairment emerged whereby recent life was most commonly impaired, followed by childhood and early adulthood, respectively. There were, however, no group-by-lifetime interactions in the ANOVA results to suggest that the pattern across lifetime periods in the TBI group differed significantly from the pattern in the HC group.
Negative temporal gradients in retrograde amnesia are typically observed when damage is more restricted to the MTLs.
31 The flat gradient observed across lifetime periods in the current study therefore is not out of keeping with diffuse brain injury that occurs as a result of TBI. Indeed, volume loss in frontal, temporal, parietal, and occipital brain regions has recently been shown to be associated with episodic autobiographical memory deficits based on structural MRI.
5 Functional imaging studies investigating both components of autobiographical memory are needed to further understand this pattern of deficits at a neural level.
It should also be acknowledged that the nature of the AMI might also contribute to the lack of a significant difference between personal semantic and episodic memory performance. The AMI does not inquire specifically about aspects of autonoetic consciousness,
12,32 such as the experience of reliving the event, thought to be characteristic of truly episodic memory.
33,34 In this study, we decided to increase the level of detail in scoring without lengthening the interview to maximize participant engagement and the sample’s representativeness. Regardless of the difference between schedules, however, it was evident in the current sample that personal semantic memory function was clearly not intact. This finding supports those of Coste et al.’s studies
4,13 demonstrating deficits in episodic and semantic aspects of autobiographical memory retrieval in more chronic and severe TBI.
On the basis of past research, notably the study by Esopenko and Levine,
5 it was hypothesized that people with more severe injuries may be more likely to have persisting deficits. The TBI group was therefore subdivided by injury severity with a cutoff at 14 days of PTA. On the PS Schedule, both TBI groups performed more poorly than HCs, but they did not differ significantly from each other. The pattern of change over time in the less severely injured group did not differ significantly from the pattern among HCs. The more severely injured group, however, showed greater improvement in the recall of childhood autobiographical facts from initial to follow-up assessment. TBI participants with more severe injuries performed more poorly, on average, than the less severely injured group following PTA emergence, but they appeared to “catch up” with the less severely injured group at follow-up. Imaging data would be needed to verify whether this reflects more widespread intracortical damage in the more severely injured group initially, with some recovery or reorganization of neural pathways over time. It is interesting to note that the different AMI subscales appeared to be more highly intercorrelated in the TBI group at the initial interview (see
Table 4). By the time of follow-up (see
Table 5), however, the intercorrelations were more similar to those of the HCs, which perhaps reflects some degree of restoration of “normal” memory pathways.
The two TBI groups also performed significantly more poorly than the HC group on the AI Schedule, although again they did not differ significantly from each other. For this schedule, there was no significant difference in the pattern of change across time between groups. The trajectory toward some improvement in the less severely injured group for recent life is worth noting in the context of Esopenko and Levine’s
5 findings of episodic autobiographical memory deficits only among those with severe TBI at 1 year postinjury. It is possible that the observation in the present study is reflective of the early stages of improvement, although longitudinal extension would be needed to support this supposition.
With respect to the third hypothesis, it was predicted that anterograde memory performance would be more closely associated with recent retrograde autobiographical memory function than more remote lifetime periods. Indeed, the only significant correlations were between the recent life section of the PS and AI Schedules and total learning across trials on the RAVLT (as well as delayed recall, in the case of the initial interview for the PS Schedule). Studies in other syndromes also have reported stronger trends between anterograde memory function and more recent than remote lifetime periods,
20,21 although rarely are these correlations particularly strong.
A strength of the current study is the knowledge of the time of onset of anterograde memory problems (i.e., date of injury); in other conditions, such as epilepsy and dementia, it is possible that retrograde memory retrieval from the recent past is influenced by the onset of anterograde memory difficulties. Furthermore, this study is unique in examining the change in both measures over time. Change in the recent life section of the PS Schedule was significantly positively correlated with change in total learning on the RAVLT. Although functional imaging data would be necessary to make inferences regarding the mechanisms underlying both processes, one hypothesis could be that this corresponding change represents the “hippocampal component” suggested by Schmidtke and Vollmer
21 to be involved in both recent retrograde memory retrieval and anterograde memory function. However, both frontal and parietal regions have also been shown to be important for the performance of list-learning tasks following TBI,
35,36 and thus the contribution of regions outside of the MTL should not be discounted.
In considering why these associations emerged for the PS Schedule but not the AI Schedule, it is important to acknowledge the nature of items in this section of the interview. Questions are related to a hobby or activity that the individual engaged in immediately prior to the injury, address and acquaintances at the time of injury (or first interview, for controls), where and with whom the individual spent the previous Christmas, and the time and location of a recent holiday. One could argue that these questions have episodic features in inquiring about specific events, times, and locations, reminiscent of what Grilli and Verfaellie
37 termed “experience-near” forms of personal semantic memory, which are thought to rely more heavily on the MTL for retrieval. This is supported by the intercorrelations among AMI measures reported in
Tables 4 and
5. Particularly in the TBI group, but also in the HC group, the performance on the recent life section of the PS Schedule was closely associated with performance on the more recent aspects of the AI Schedule.
The findings drawn from the present study relied on follow-up data obtained over the telephone from a smaller group of participants than initially entered the study. The group lost to follow-up scored higher on the AI Schedule than the remaining sample, which might have restricted the range of scores and therefore the strength of the correlation observed with community integration. Difficulty with follow-up in this population is prevalent, particularly at the more acute stages post-TBI, as has been directly acknowledged in some studies.
38 The TBI group was inevitably much more heterogeneous than the HC group, and it should be acknowledged that the pattern of change was highly variable among individuals. Other factors, such as mood, which was not assessed given concerns surrounding the length of the interview at follow-up, could also have contributed to intraindividual differences over time. Controlling for such variables in future studies, and also including other measures of anterograde memory and cognitive functioning, might strengthen the statistical findings. Lastly, the reader should be reminded that this sample included only individuals with moderate-severe injuries and excluded individuals with severe psychiatric or substance-use history, who might have had preexisting autobiographical memory deficits.
The autobiographical memory deficits found in the present study, taken together with the suggestion of an association with community integration, suggest that finding ways to support autobiographical memory function in rehabilitation may be of value. Past research suggests that training individuals with TBI to utilize AMs can benefit performance on a planning task.
39 Incorporating some form of reminiscence therapy
40 into rehabilitation may therefore be of value in relation to the directive function of autobiographical memory. It is possible that there may also be parallel benefits with respect to social communication and personal identity following TBI, given that autobiographical memory also serves “social” and “self” functions.
41 It is important to note that memories that are not reinforced or retrieved are more likely to be forgotten.
42 Encouraging family members to discuss past experiences with the individual with TBI therefore seems important to minimize the possibility that these memories will be lost over time.
In summary, autobiographical memory deficits were common following moderate-severe TBI and showed limited improvement over the first 6 months after emergence from PTA. This deficit extended across all lifetime periods for both more episodic and semantic autobiographical memory types. Individuals with more than 14 days PTA performed more poorly in the recall of personal facts from childhood after PTA emergence but improved to the level of the shorter PTA group at the 6-month follow-up. The retrieval of recent retrograde autobiographical memory was more closely associated with anterograde memory function than more remote memories, and some overlap in underlying mechanisms was supported by concurrent change over time. The moderate association between aspects of autobiographical memory functioning and community integration suggests that future research exploring the functions of autobiographical memory in TBI may be of value. Clinicians should be mindful that although it may be possible to delimit, using the patient’s last memory report, a relatively brief period prior to injury for which the patient has no memory, there is a much more far-reaching retrieval deficit that is largely missed in standard neuropsychological assessment.