Traumatic brain injury (TBI) is a serious cause of mortality and disability worldwide. It is estimated that 69 million individuals worldwide sustain TBIs each year (
1). In the European Union, 56,946 TBI-related deaths and 1,445,526 hospital discharges were estimated in 2012 (
2). The most common signs and symptoms of TBI include headache, nausea, focal neurologic deficits, and confusion (
3).
Delirium is a neurobehavioral syndrome that is described as abrupt and transient impairment of attention, consciousness, and behavior (
4). Delirium is a common complication of patients with TBI, with reported incidence rates ranging from 46% to 69% (
5,
6). Alterations of neurotransmitter synthesis, function, and availability mediate complex behavioral and cognitive changes. Dopamine is a commonly implicated neurotransmitter in delirium (
4). Dopamine levels have been shown to rise in acute TBI across numerous brain regions, including the brainstem, striatum, hypothalamus, and medial prefrontal cortex (
7). Impaired functioning of these brain regions, especially in the nondominant hemisphere, was implicated in the delirium risk. However, the increase of dopamine levels was short-lived and was followed by a hypodopaminergic state (
7,
8). The major enzyme that degrades the excess of dopamine in the prefrontal cortex (PFC) is catechol-
o-methyltransferase (COMT) (
9).
A common genetic variation of the
COMT gene is the Val
158Met (rs4680) single nucleotide polymorphism, in which a single G/A base-pair substitution leads to a valine (Val) to methionine (Met) substitution at codon 158 (
10). This Met substitution significantly reduces functional activity of the COMT enzyme to one-quarter of its activity encoded by the Val allele. Therefore, the Met carriers have greater extracellular dopamine level in the PFC (
11).
COMT is highly active in the PFC, a brain region that is associated with executive and cognitive functions (e.g., attention, memory, visuospatial ability, orientation), which are notably impaired in delirium patients (
5). Given that PFC modulates striatum activity, the reduced dopamine levels in PFC result in greater striatum dopamine release that in turn is associated with increased psychosis risk (
12). In previous studies, increased COMT activity in the Val
158 carriers was linked to greater risk of schizophrenia and psychosis-related endophenotypes, such as worse working memory and cognitive flexibility as well as neurostructural alterations (
13,
14). Hence, we hypothesized that the Val
158 allele could be associated with greater delirium risk. However, whether the COMT Val
158Met polymorphism influences delirium following brain injury has yet to be studied.
Previous studies investigated the association of the COMT Val
158Met genotype with functional and cognitive outcomes after TBI. The dopamine system in the PFC is strongly associated with cognitive functioning, especially attention and working memory (
15). The Met
158 allele was previously described in the literature as a predictor of better performance in working memory and attention tasks (
16). In another study it was shown that the Val
158/Val
158 genotype might be associated with greater impairment of certain cognitive domains, such as modulation of impulsive response (
17). However, there is uncertainty about association of the
COMT gene polymorphism with functional outcome. One study found that the Met
158 allele was associated with better functional outcomes 6 months after TBI; other studies failed to replicate this association (
18,
19).
In the present study we investigated the association of the COMT genetic polymorphism with delirium risk, functional outcome, and cognitive outcome of patients with complicated mild to moderate TBI. Because Met158 carriers have reduced COMT enzyme activity and therefore higher PFC dopamine levels, we hypothesized that this allele could be associated with the lower risk of delirium because of an inhibitory effect on striatum dopamine release and synthesis.
Methods
Study Design
This prospective observational cohort study was carried out between October 2017 and May 2018 at the Neurosurgery Department of the Lithuanian University of Health Sciences Hospital Kauno Klinikos, Lithuania. The study was approved by the Ethics Committee of the Faculty of Medicine, Lithuanian University of Health Sciences. Informed consent was obtained from all study patients before enrollment in the study. For patients who were unable to provide consent due to their injury, consent was obtained from their legally authorized representative, and these patients were reconsented once they became cognitively able at a later inpatient follow-up assessment.
Assessment of delirium symptoms was performed within 24 hours after admission to the neurosurgical ward. All patients were reevaluated for delirium symptoms and level of consciousness for 4 consecutive days. Functional and cognitive outcomes were evaluated at the day of hospital discharge. All assessments were performed by trained study researchers: a medical doctor and a senior medical student (M.K. and D.N.). Demographic and clinical data, as well as information about alcohol consumption, were collected during follow-up visits.
Patient Selection
Adult patients admitted for inpatient treatment were included in the study. Our analysis was restricted to patients with complicated mild to moderate TBI (
20,
21). Complicated mild TBI was defined by Glasgow Coma Scale (GCS) score ranging from 13 to 15 at admission with radiographic evidence of brain lesion, or depressed/linear skull fracture, or both. Moderate TBI was defined by an admission GCS score ranging from 9 to 12 with positive radiological findings of brain injury or skull fracture. In our center, patients with moderate TBI or with mild TBI and documented brain parenchymal injury or skull fracture are admitted for inpatient observation and treatment at the neurosurgery department. Acute intracranial neuroimaging abnormalities of brain injury included skull fractures, acute intracranial pathology (contusions/hemorrhage, epidural hematoma, acute subdural hematoma, or traumatic subarachnoid hemorrhage), or both. Patients discharged before the end of the follow-up period or transferred to other departments or hospitals were excluded from the analysis.
Biospecimen Collection and Genotyping
Buccal epithelium samples for genomic DNA extraction were collected using cotton swabs and rinsed into tubes containing phosphate-buffered saline. DNA was extracted using the PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, Calif.). The isolated DNA was quantified with the Nano-Drop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, Mass.). Extracted DNA was preserved at –20°C until genotyping. The COMT rs4680 polymorphism was genotyped using polymerase chain reaction (PCR) with Applied Biosystems 7500 Real-Time PCR system (Thermo Fisher Scientific) following manufacturer’s guidelines and using predesigned TaqMan Assay. Single allele polymorphism of the COMT rs4680 gene was assessed with the Applied Biosystems 7500 Software, version 2.0.5 (Thermo Fisher Scientific). For genotyping quality control, 10% of samples were regenotyped with 100% compliance.
Neuropsychiatric Assessment and Outcome Parameters
Delirium.
The presence of delirium was evaluated daily using the Confusion Assessment Method (CAM) (
5). Follow-up duration of 4 days was chosen according to previous studies (
5,
22). The CAM is the most widely used standardized delirium assessment instrument for clinical and research purposes. A systematic review showed the CAM’s sensitivity of 43%−100% and specificity of 63%−100% for delirium (
23). The delirium diagnostic algorithm is based on four cardinal features of delirium: acute onset and fluctuating course, inattention, disorganized thinking, and altered level of consciousness. According to the CAM, diagnosis of delirium requires the presence of the first two features, with the addition of either disorganized thinking or altered level of consciousness (
23).
Functional outcomes.
Functional outcomes were evaluated using the Glasgow Outcome at Discharge Scale (GODS), which is designed to assess disability after brain injury in inpatient setting (
24). GODS provides an overall measure of disability on the basis of information obtained through structured interview focused on cognition, independence, ability to work, social interaction, and intellectual interest. It is derived from the Extended Glasgow Outcome Scale and has the same outcome categories: 1=dead, 2=vegetative state, 3=lower severe disability, 4=upper severe disability, 5=lower moderate disability, 6=upper moderate disability, 7=lower good recovery, and 8=upper good recovery. GODS is an indicator of prognosis after TBI based on strong associations with other functional outcome scales (
25).
Cognitive outcomes.
The Montreal Cognitive Assessment (MoCA) scale is designed to detect mild cognitive impairment and is a valid tool for assessment of gross cognitive function disturbance in brain injury patients with intracranial hemorrhage (
26). The MoCA is a 30-point test that evaluates seven cognitive domains: visuospatial/executive functions, naming, verbal memory registration and learning, attention, abstraction, 5-minute delayed verbal recall, and orientation.
Alcohol abuse.
Alcohol misuse risk was evaluated using the Alcohol Use Disorders Identification Test (AUDIT) (
27). The AUDIT scale has 10 questions (with a maximal possible score of 40) that evaluate frequency and amount of drinking alcohol beverages, drinking behavior, dependence symptoms, and alcohol-related harm. Eight or more points on the AUDIT indicate a risk of alcohol use disorder. We divided patients into medium or low (score 0–15) and high or very high alcohol misuse risk (score 16–40) (
28). The AUDIT was applied via structural patient interview.
Statistical Analysis
Group differences of baseline descriptors were assessed using the Pearson’s chi-square test for categorical variables and nonparametric one-way analysis of variance test for interval data. Post hoc analyses using the chi-square were used to assess differences in categorical variables with cell count greater than three.
For the purpose of evaluating potential protective benefit of the Met
158 allele, Met
158/Met
158 and Met
158/Val
158 were combined as a single group as previously described for COMT in order to investigate COMT’s genotype role for delirium risk and functional and cognitive outcomes after TBI (
19,
29). Other clinical predictors of delirium and outcomes after TBI were selected on the basis of prior published reports to help control for potential cofounders in multivariable analyses. Binary logistic regression was performed with delirium as the dependent variable and COMT genotype, preexisting neurological disorder, alcohol abuse, age, and GCS score <15 as predictor variables (
5,
30). We chose GCS score and age as the most relevant predictors of functional outcomes after TBI (
19,
31). Ordinal logistic regression was performed with GODS score as the response and COMT genotype, age, GCS on admission, and delirium as predictor variables. Multivariate linear regression analysis (enter method) was conducted to determine predictors of cognitive functions (total MoCA score) with COMT genotype, age, education, GCS on admission, and delirium as predictor variables (
32). All multivariable regression models conformed to the test for goodness of fit. The Nagelkerke pseudo-R
2 (NR
2) was used to estimate the variance explained by the model.
Statistical analysis was carried out using SPSS, version 25 (IBM, Armonk, N.Y.). All calculated p values were two-tailed, and statistical significance was set at p<0.05.
Results
Demographic and Clinical Descriptors
Eighty-nine patients admitted for inpatient treatment for complicated mild to moderate TBI were included in the study (
Table 1). The mean age of the study patients was 56 years and the majority of patients were male (81%). The
COMT genotype distribution was 26% for the Met
158/Met
158 (N=23), 54% for the Met
158/Val
158 (N=48) and 20% for the Val
158/Val
158 (N=18) polymorphism. The COMT allelic frequencies (A=0.53, G=0.47) did not deviate from the Hardy–Weinberg equilibrium (χ
2=0.6, p=0.439). The greater prevalence of patients with preexisting neurological disorder (p<0.05) was in the Val
158/Val
158 homozygotes in comparison with the Met
158/Met
158 or Val
158/Met
158 carriers. The risk of alcohol abuse was lower in the Val
158/Val
158 homozygotes when compared to the other two groups (p<0.05) (
Table 1).
Other demographic and clinical characteristics were similar across the three studied genotypes. Trauma severity characteristics, including posttraumatic amnesia duration, loss of consciousness duration, and GCS score, were similar (p>0.05) across the three groups (
Table 1).
COMT Genotype and Delirium Risk
Seventeen (19%) patients were diagnosed with delirium. Delirium incidence rate was greater in the Val158/Val158 homozygotes (38,9%) when compared with Met158 carriers (14,1%; χ2=5.72, df=1, p=0.017).
In univariate regression analyses, the COMT Val
158/Val
158 genotype and GCS score <15 were associated with increased risk of delirium (p=0.025 and p=0.001, respectively;
Table 2). In multivariable regression analyses adjusted for alcohol misuse, histories of neurological disorders, and patient age, the COMT Val
158/Val
158 genotype and GCS score <15 remained associated with elevated delirium risk (odds ratio=4.57, 95% CI=1.11, 18.9, p=0.036, and odds ratio=6.68, 95% CI=1.96, 22.7, p=0.002, respectively) (
Table 2). The multivariable model was statistically significant (p=0.003) and explained 30% of variability in delirium.
COMT Genotype and Functional Outcome
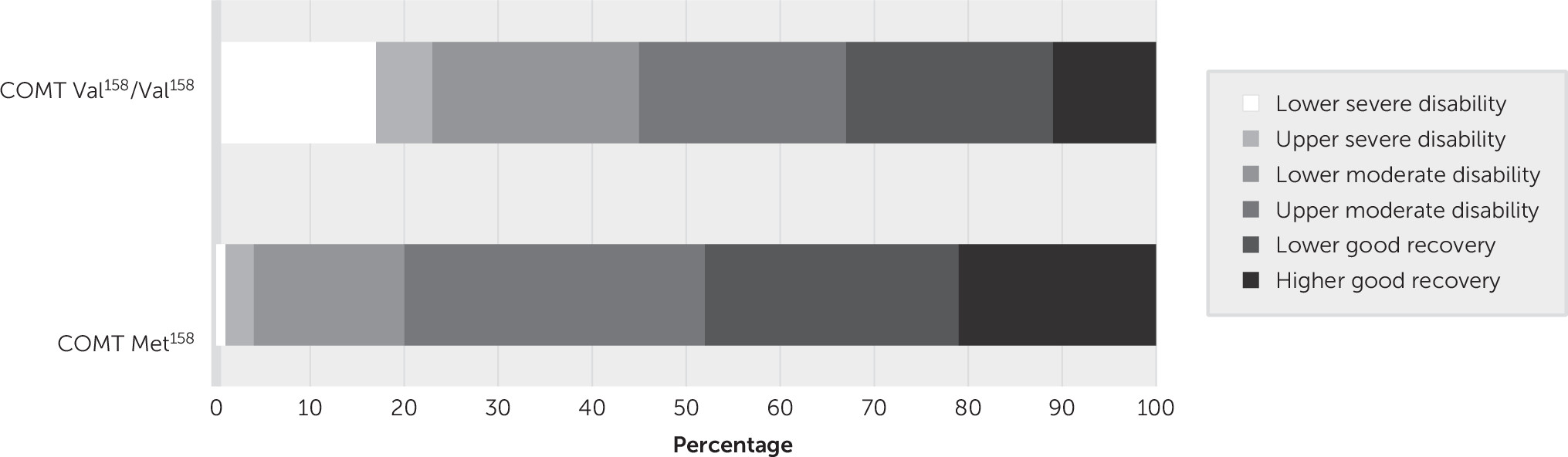
The GODS scores as a function of COMT genotype (Val
158/Val
158 versus Met
158 carriers) are presented in
Figure 1. The proportion of patients with lower severe disability (GODS score of 3) was greater in the Val
158/Val
158 homozygotes relative to the Met
158 carriers (17% and 1% respectively; p<0.05). The total GODS score was significantly lower in the Val
158/Val
158 homozygotes than in the Met
158 carriers (mean, 5.61 [SD=1.61] versus 6.44 [SD=1.15], respectively; F=3.85, df=1, 87, p<0.05).
The COMT Met
158 allele was associated with higher GODS scores at discharge in univariate regression analysis (odds ratio=2.82, 95% CI=1.10, 7.27, p=0.031) (
Table 3). In multivariate regression analysis, worse functional outcome was associated with older age and presence of delirium (odds ratio=0.97, 95% CI=0.95, 0.99, p=0.008, and odds ratio=0.04, 95% CI=0.01–0.16, p<0.001, respectively) (
Table 3). The COMT and GCS score of 15 lost their predictive value when age and delirium were included in the analysis (p=0.338 and p=0.087, respectively). The model was statistically significant (p<0.001) and explained 44.5% of the variance.
Predictors of Cognitive Status at Discharge as Measured by the MoCA
There were no significant differences between Val
158/Val
158, Val
158/Met
158, and Met
158/Met
158 genotypes in cognitive functioning as measured using the MoCA overall, attention, and memory scores (F=4.64, df=2, 73, p=0.098; F=5.1, df=2, 73, p=0.078; F=3.81, df=2, 73, p=0.149; respectively) (
Table 1).
The COMT Val
158/Val
158 genotype was not associated with cognitive outcome in univariate (p=0.39) and multivariate regression analyses (p=0.588). Delirium and older age were associated with lower MoCA score at discharge in univariate regression analyses (p<0.001 and p=0.035, respectively) and multivariate (p=0.038 and p=0.015, respectively) regression analyses (
Table 4). More than 12 years of education and higher GCS score were associated with better cognitive outcomes in univariate (p=0.027 and p=0.001, respectively) and multivariate (p=0.024 and p=0.034, respectively) regression analyses (
Table 4).
Discussion
We found that Val158/Val158 genotype carriers were at elevated delirium risk relative to Met158 carriers. However, the COMT genotype was not associated with functional and cognitive outcomes in multivariate analyses. Delirium was associated with unfavorable functional and cognitive outcome adjusting for demographic characteristics and disease severity.
To the best of our knowledge, this is the first study of a possible association of COMT polymorphism with delirium risk in patients with TBI. The role of COMT in the development of delirium remains unclear. We found only one previous study that failed to show the association between the COMT genotype and delirium risk in hospitalized elderly patients (age >65 years) after hip fracture (
33). Our findings suggest that the COMT genetic polymorphism can be a valuable biomarker of delirium risk in patients with TBI. Other dopamine metabolism related genes, such as the Solute Carrier Family 6 Member 3 and dopamine receptor D
2, were studied in relation to delirium in non-TBI settings (
34). Studies exploring a possible role of dopamine metabolism dysregulation in TBI disease course and prognosis are encouraged to identify TBI patients at elevated risk for unfavorable outcome.
We did not find an association of COMT genetic polymorphism with functional and cognitive outcomes at discharge. Previous studies of patients with TBI examined the association of the COMT genetic polymorphism with long-term outcomes. For example, a study of patients with moderate to severe TBI did not find a statistically significant difference between genotypes and functional or cognitive outcome at the 12- and 24-month follow-up assessments (
18). In contrast, other studies found that the COMT Met
158 allele was associated with better functional outcomes after uncomplicated mild TBI at 6 months following the injury (
19). However, there was no significant difference in functional outcomes between genotypes in adjusted analyses. We postulate that worse outcomes could be due to the association between COMT genotype and posttraumatic stress disorder, which was a significant predictor of functional outcomes in multivariable analysis. Our results suggest that COMT’s association with delirium may influence worse outcome of TBI patients. The COMT genetic polymorphisms may predispose patients to develop delirium and in turn predict unfavorable prognosis.
The association of worse cognitive outcome with the COMT Val
158/Val
158 genotype may be due to decreased dopamine levels (
35). COMT Val
158/Val
158 is associated with more perseverative response after mild TBI (
17). Another study found that the COMT Val
158/Val
158 polymorphism was associated with worse nonverbal processing speed (
36). Collectively, these data suggest that ability to concentrate and manage executive functions is related to the COMT genotype. However, the latter studies measured cognitive functioning at least 6 months after TBI, whereas our study was designed to investigate cognitive functioning in an acute TBI setting.
The incidence rate of delirium (19%) in our study was lower than previously described in the literature. For example, Nakase-Thompson et al. (
6) diagnosed delirium in 69% of patients with TBI, whereas in Maneewong et al.’s (
5) study the delirium incidence rate was 46.3% (
5,
6). It is possible that the lower prevalence rate of delirium can be attributed to less severe TBI in our study.
We found that incident delirium was associated with greater risk of unfavorable functional and cognitive outcomes. To the best of our knowledge, there are no previous studies investigating the prognostic role of delirium in patients with TBI. In surgical patients, delirium was associated with longer hospital and intensive care unit stays, greater likelihood of discharge to nursing facilities, and greater need for inpatient physical therapy (
37). Previous studies also showed that delirium in hospitalized patients was associated with cognitive impairment (
38,
39). TBI patients who develop delirium should be considered at elevated risk for unfavorable outcomes. Delirium should be identified in a timely way and appropriately managed in patients with TBI.
This study has limitations. As a result of a relatively small sample size and consequentially small statistical power, false negative findings cannot be ruled out. Secondly, only three patients had moderate TBI, thus limiting generalizability of our findings to moderate TBI. Our results cannot be generalized to patients with severe TBI and those with mild TBI without focal brain lesions, since these were the study exclusion criteria. There is also a need to examine gene-gene interaction in susceptible loci in the context of TBI to better understand mechanisms through which COMT’s molecular pathway may influence delirium risk and TBI prognosis.
Conclusions
In patients with complicated mild to moderate TBI, the COMT Val158/Val158 variant is associated with elevated risk of delirium when compared with the Met158 carriers. The COMT genotype is not associated with functional and cognitive outcomes in multivariable analyses. Delirium is associated with unfavorable functional and cognitive outcome independently from demographic characteristics and TBI severity. Studies with larger samples of patients with TBI are warranted to identify risk factors for optimal assessment and management strategies of delirium in TBI patients.