A complex ion has a coordinating metal ion surrounded by other ions in Lewis acid-base interactions. The surrounding ligands form coordination bonds, such that all ligands donate a pair of electrons to the bond and thus function as Lewis bases. There is a very broad group of compounds that meet this definition with multitudes of human exposures, making generalizations difficult. The chemistry of complex metal ions encountered in medicine is diverse. For example, the chemotherapy agent cisplatinum [Pt(NH3)2Cl2] is considered simple. In contrast, cyclical chelates such as gadoteric acid [C16H25GdN4O8] are considered complex. Some agents, such as cyanide, only become a complex metal ion after introduction into living cells.
Cyanide
Few poisons are more infamous than cyanide. It is highly lethal, and survival can be limited in acute exposures (
14). Cyanide compounds have very narrow medical use. Iatrogenic cyanide poisoning can occur with the intravenous administration of nitroprusside, particularly in patients with renal failure, as a result of the impaired excretion of thiocyanate (
4,
15,
16). Tragic intentional uses of cyanide compounds include mass suicide (potassium cyanide was used in the Jonestown mass suicide) and homicide (hydrogen cyanide was used in the killing center of Auschwitz-Birkenau, the largest killing center of the Nazi regime) (
17,
18). Occupational cyanide exposures include electroplating and fumigation (
19). In Western countries, the most common source of cyanide poisoning is accidental due to smoke inhalation from fires (
20). Hydrogen cyanide is produced when materials containing nitrogen (e.g., plastics, wool, vinyl, and wood) undergo combustion (
21). This is frequently in combination with carbon monoxide exposure, a more widely recognized source of morbidity and mortality in smoke inhalation. Data support the role of cyanide in smoke inhalation injury, with toxic levels found in 33%−90% of victims who die in a closed-space fire (
20).
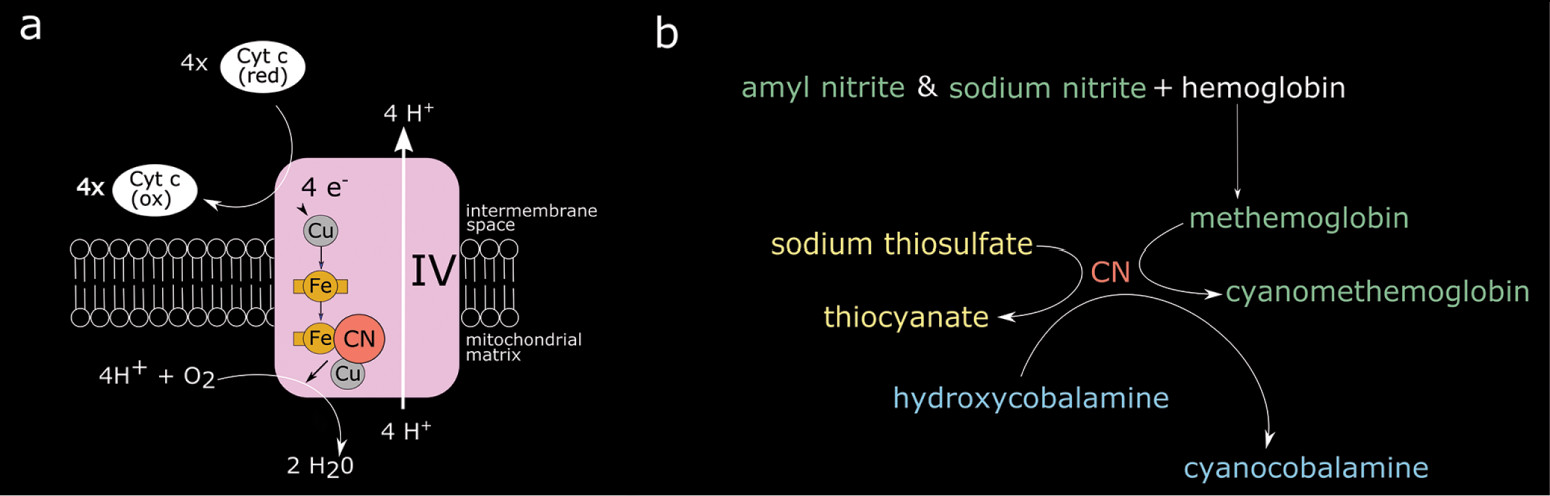
When cyanide is introduced to the human body, it diffuses into cells and binds at the binuclear (iron[Fe]/copper[Cu]) center of cytochrome a3 complex IV in the electron transport chain (
Figure 2) (
3,
4). This leads to cessation of adenosine triphosphate (ATP) production, profound cellular hypoxia and a shift to glycolytic metabolism that results in lactic acidosis. Acute toxicity can be divided into early manifestations (within minutes) that include neurologic (anxiety, confusion, headache), respiratory (tachypnea), and cardiovascular (tachycardia) symptoms, and later manifestations occurring with the progression of hypoxia include seizures, coma, hypotension, arrhythmias, and death (
4). Treatment includes supportive measures (i.e., oxygen administration and intubation) and administration of a cyanide antidote. Two approaches are approved by the Food and Drug Administration for acute cyanide poisoning (
Figure 2). The cyanide antidote kit contains sodium and amyl nitrite and sodium thiosulfate. The nitrites induce the production of a preferential cyanide binding site (methemoglobin), while sodium thiosulfate combines with cyanide to generate thiocyanate which is then renally excreted. The more recently approved (in 2006) hydroxycobalamin reacts with cyanide to produce cyanocobalamin (vitamin B12) (
4).
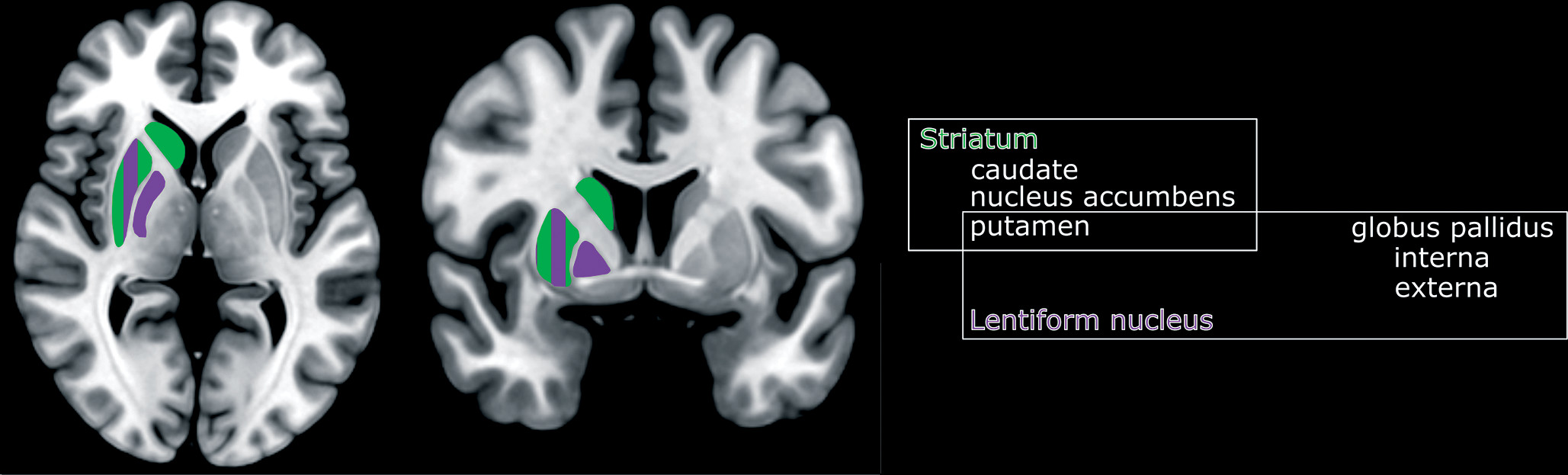
If patients survive the acute toxicity of cyanide, neuropsychiatric sequalae are predicated on injury to brain structures that have high oxygen and glucose requirements, such as the basal ganglia (
Figures 1 and
3). Parkinsonism and dystonia are common sequalae in those that survive acute cyanide toxicity (
13,
22,
23). Cyanide poisoning most commonly presents with hyperintensities in the basal ganglia (particularly in the bilateral putamen) on MRI. With more significant exposures, there can be hemorrhagic necrosis and cellular death in the cortex and cerebellum (
12,
13,
24). Surprisingly, although the hippocampus is a highly oxygen-sensitive structure, published case reports have not commonly found hippocampal injury after acute cyanide toxicity (
13,
22,
23,
25–
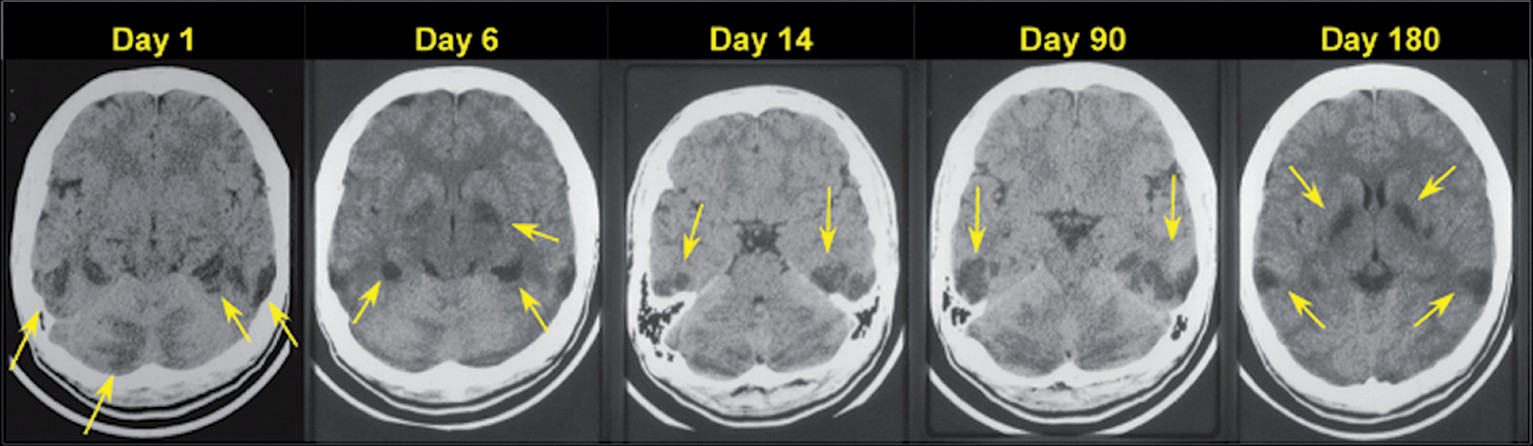
29) The lesions may not appear immediately on computed tomography (CT) (
Figure 3). They can take several weeks to months to appear as the acute initial edema from hypoxia resolves with some resolution of the subacute hypertensities in weeks to months (
30). Of note, the severity and chronicity of the clinical symptoms do not always match to the imaging findings (
31).
Chronic, low-level cyanide exposure can result in toxicity as well. The cyanogenic shrub, Cassava (
Manihot esculenta), is a food staple for hundreds of millions of humans world-wide, especially in Africa (
32). Cyanogenic compounds can be removed from the cassava plant through processing methods. Inadequate processing in times of social or environmental adversity can result in retained cyanogenic compounds and thus human exposure when consumed (
32). Two diseases in humans have been associated with Cassava consumption: tropical ataxic neuropathy (TAN) and konzo (meaning “tired legs” in Kiyaka, a dialect spoken in the Democratic Republic of Congo) (
33,
34). Both are myeloneuropathies endemic throughout the tropics. Although chronic, low-level cyanide toxicity is a commonly proposed mechanism given the association with consumption of cassava (
32,
33,
35), some authors have disputed the importance of cyanide in the pathophysiology of both diseases (
36). TAN is a sensory-predominant myeloneuropathy which presents with distal neuropathic pain, severe loss of dorsal column sensory modalities, diminished deep-tendon reflexes in the legs, and preservation of strength (
34). In contrast, konzo is a predominantly a motor myeloneuropathy with irreversible paraparesis (and rarely, tetraparesis), that may also be accompanied by paresthesias and visual loss that tend to improve over time (
33). Cognitive deficits have also been documented in children with konzo (
37). Interestingly, low-level cyanide toxicity has also been hypothesized to play a role in two visual disorders: tobacco amblyopia and Lieber’s hereditary neuropathy (
38,
39).
Cisplatinum
Metal-based compounds are frequently used as antineoplastic agents. Cisplatinum is a prototypical metal-based antineoplastic agent that has served as the foundation for the synthesis of other organometallic compounds used in cancer therapy (
40). It is a complex metal ion with a relatively simple structure composed of a central platinum molecule with two ammine molecules and two chlorine atoms attached. Once cisplatinum is transported into cells, it is hydrolyzed into an electrophilic compound that binds to purine residues on DNA, leading to inhibition of cellular division and apoptotic cellular death. This mechanism is responsible for both the therapeutic cytotoxic effects on neoplasms as well as the toxicity and side effects. It has been used alone or in combination with other chemotherapeutic agents and is an example of a complex metal ion that has significant life-saving medical uses. Nevertheless, adverse effects are common, with over 70% of patients experiencing gastrointestinal symptoms, and up to 50% experiencing neurologic symptoms (most commonly, peripheral neuropathy) over the course of their treatment (
41,
42). Hepatic, renal, and cardiac toxicity are all well-described side effects of cisplatinum therapy (
43).
Platinum-based agents, including cisplatinum, can lead to significant neurotoxicity (
44). Sensory neuropathy is the most common dose-dependent neurotoxic effect. In a study of patients undergoing treatment for metastatic germ cell tumors, 11% had paresthesias after 3–4 cycles of chemotherapy with cisplatinium, with 65% experiencing paresthesias at 3 months (
45). Fortunately, the majority of patients experienced improvement, with only 17% having persistent symptoms at one year after completion of therapy. Ototoxicity leading to significant hearing loss and tinnitus are well-described toxicities related to cisplatinum especially in children (
46). As in neuropathy, ototoxicity is dose-dependent. In one study, 80% of adults with germ cell tumors experienced a hearing off of at least 20 dB (
47). A study of children receiving cisplatinum for hepatoblastoma found 63% experienced of at least Brock grade 1 or higher loss (40 dB at a minimum of 8 kHz) (
48).
CNS toxicity is much less common but has been described with cisplatinum. A subacute encephalopathy with headaches, severe hypertension, cortical blindness, and seizures has been described, and most likely represents posterior reversible encephalopathy syndrome (PRES; sometimes referred to as reversible posterior leukoencephalopathy syndrome) (
49–
55). The most common MRI findings are hyperintensities on T
2 weighted images, likely indicating edema, primarily in the fiber tracts of the parietal lobe, occipital lobe, or periventricular areas. Other less commonly cited edematous areas include frontal and temporal lobes or the subcortical regions. Unlike the injury of cyanide, there is usually resolution of this syndrome with treatment and thus a decrease in the white matter edema. The MRI images show normalization and decreased hyperintense signal within a few weeks to months (
51,
54). The incidence of PRES in patients treated with cisplatinum is not known. A review of all cases of PRES between 2005 and 2011 at one site (Memorial Sloan Kettering Cancer Center) found that 55% of patients (N=17/31) had received chemotherapy within the prior month (
56). Of these, two had received carboplatin/paclitaxel combination therapy, two carboplatin monotherapy, and two oxaliplatin monotherapy. Data on the number of patients given a platinum-based compound who did not develop PRES is not available, but expected to be quite large, leading to a low estimated incidence.
Newer MRI techniques are beginning to be applied to examine the long-term effects of cisplatinum upon brain networks and cognitive function. One group used diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI) in combination with neuropsychological testing to examine tissue microstructure in a cross-sectional study of testicular cancer (TC) patients with (N=27) and without (N=18) cisplatinum-based chemotherapy 14 years after treatment (
57). Although the cisplatinum-treated group had both lower cognitive performance and higher radial kurtosis in multiple white matter areas (corona radiata, bilateral internal capsules, bilateral superior longitudinal fasciculi, corpus callosum) than the group without chemotherapy, imaging findings were not related to cognitive performance. A different group used DTI in combination with mathematical graph analysis to assess structural network characteristics in a longitudinal study of TC patients with (N=22) and without (N=42) cisplatinum-based chemotherapy at baseline (3 months after orchiectomy) and 6 months later (3 months post completion of the chemotherapy) (
58). Results indicated that the cisplatinum group had decreased network and local efficiencies, as well as decreased small-worldness, all associated with decreased cognitive performance (
58). However, in both these studies, it is challenging to affirm that the declining cognitive performance was solely due to the cisplatinum, as other agents in the cisplatinum-based therapy have been associated with possible cognitive effects (
57).
Gadolinium-Based Contrast Agents
Gadolinium-based contrast agents (GBCAs) consist of a central gadolinium ion (Gd3+) that is chelated to produce a stable structure, an important feature as free gadolinium ions are highly toxic. GBCAs are examples of complex metal ions with significant medical diagnostic value and rare adverse effects (
59). Gadolinium is a paramagnetic molecule that reduces T
1-relaxation time, thereby generating high signal on T
1-weighted MRI (
60). GBCAs are characterized by their structure and charge: each compound can be either linear or macrocyclic and ionic or nonionic.
The most feared toxicity from GBCAs is nephrogenic systemic fibrosis (NSF; also known as nephrogenic sclerosing dermopathy), a rare but potentially fatal complication. The pathophysiology of NSF is driven primarily by fibroblast stimulation, leading to the characteristic changes to skin and injury to other organs (
61). The skin thickens and hardens in the extremities, sometimes involving the trunk, but usually sparing the face. The morbidity is high, with contractures developing, and sometimes involving organ systems beyond the skin, including the muscle, heart, and lungs (
62). In a multicenter retrospective study of 83,121 patients, 15 were identified with NSF, all of whom had received a greater than standard dose of GBCA (
63). The incidence with high dose GBCA in patients on chronic hemodialysis was 0.4%, and 8.8% when restricted to those with an estimated glomerular filtration rate of less than 15 mL/min who were not in hemodialysis. More recently, a systematic review and meta-analysis of patients with stage 4 or 5 chronic kidney disease (pooled sample of 4931) who were administered a group II GBCA (e.g., gadobenate dimeglumine, gadobutrol), found zero patients with NSF. The upper bound of the 95% confidence interval was 0.07%, suggesting that incidence using group II GBCAs is quite low (
64).
While the clinical significance remains in question, administration of GBCAs has been shown to deposit gadolinium in the brain, skin, and bones of individuals with normal renal function (
65–
68). Initial reports demonstrated gadolinium retention in deep gray matter structures (e.g., the globus pallidus and dentate nucleus) in human brain tissue based on MRI (
65,
66). There have been several systematic reviews and an international conference led by the National Institutes of Health (
69–
71). Retention of gadolinium can be visualized on unenhanced T
1-weighted MRI as increased signal intensity, most commonly in the dentate nucleus of the cerebellum and the globus pallidus, with some reports of much lesser wide-spread brain deposition (
72). This increase is most commonly found after multiple infusions of the linear contrast agents, but also to a lesser extent with the macrocyclic agents. While the reasons for highest signal in the globus pallidus and dentate remain unclear, both structures are rich in iron and are associated with multiple metal-transporter-mediated conditions (
72). Studies in rats have consistently implicated a greater degree of deposition for linear than macrocyclic GBCAs, although the human data has been less consistent (
59). This is an area of active study with new clinical recommendations including decreasing the use of linear agents, limiting the dosage of gadolinium to the least amount needed, and caution in vulnerable populations (
70).
Although “gadolinium-deposition disease” (GDD) was first proposed in 2016, it is not widely accepted as a distinct disease with a proven pathophysiology (
73,
74). Proposed symptoms should begin within two months of GBCA administration, are broad and nonspecific, and include neuropathic pain, joint stiffness, muscle spasms, buzzing sensation, fatigue, clouded mentation, and skin thickening, discoloration, and pain, although the exact criteria remain poorly described and no validation studies of any criteria have been published (
75). While GDD remains unproven, it nevertheless represents a significant medicolegal risk concern (
75) and mitigation strategies have been proposed (
59,
75).