Delirium is an acute impairment of consciousness that affects all higher cerebral-cortical functions and is common in seriously medically ill patients, with reported incidence rates between 4% and 55% among patients in intensive care units (ICUs) (
1). Increasingly recognized as commonly occurring during the COVID-19 pandemic (
2), delirium has a higher prevalence than other conditions affecting the central nervous system (CNS) when compared with stroke, encephalitis, convulsions, or meningitis (
3).
Most patients with COVID-19, caused by the SARS-CoV-2 virus, have respiratory symptoms and complications. SARS-CoV-2 enters the body via the angiotensin-converting enzyme 2 receptor present in epithelial or endothelial cells from respiratory and gastrointestinal systems, blood vessels, kidneys, heart, liver, and brain, among other organs; therefore, it has the potential to directly affect all of these tissues (
4). The virus also triggers an inflammatory response, humoral and cellular, with some patients developing an exaggerated response as a cytokine storm or autoimmune reaction (
5). Patients with severe infection develop systemic coagulopathy, with risk of thromboembolism and D-dimer elevation (
6). Hypertension, obesity, diabetes, and other cardiovascular risk factors are associated with increased severity of COVID-19 (
7).
Delirium in patients with COVID-19 has many possible etiologies, including metabolic, respiratory, and coagulation alterations that are consequences of the direct effects of SARS-CoV-2 on peripheral organs and systems. Additionally, systemic inflammation that alters the blood-brain barrier leads to a CNS immune response (
8). The virus produces a direct effect in the brain in a minority of cases (encephalitis and meningitis), such that delirium could result from a CNS infection (
9,
10) as well as brain inflammation.
There are limited reports on delirium during the COVID-19 pandemic, mostly case reports (
11–13) or retrospective studies (
3,
14), although one prospective study of two ICU cohorts in France reported a 79.5% incidence rate of delirium using the Confusion Assessment Method–ICU (CAM-ICU) (
9). COVID-19 delirium often presents as the hyperactive motor subtype in up to 69% of patients (
9,
12), although the hypoactive subtype may be more common in the elderly (
11,
14). EEGs have shown generalized slowing consistent with delirium (
15). Neuroimaging has most often found microhemorrhage with a predilection for the splenium in 60% of patients, acute and subacute infarcts in 25%, and watershed white matter hyperintensities in 20% (
3).
Medications used to manage delirium in patients with COVID-19 have included haloperidol and other antipsychotics, with melatonin as prophylaxis. Drugs used to manage hyperactivity have included haloperidol, dexmedetomidine, clonidine, trazadone, benzodiazepines, and valproic acid (
12,
16,
17). Opioids are used in ICUs to manage pain and respiratory distress, although these are also deliriogenic, as are benzodiazepines. Dexmedetomidine may be associated with a lower risk of delirium than other sedating medications used in the ICU, such as propofol (
18,
19).
To our knowledge, no reports have examined the relationship between COVID-19 disease status severity and delirium severity or a systematic clinical assessment of the relationship of etiologies with delirium in a consecutive cohort. Because delirium almost always has multifactorial precipitants, we anticipate multiple etiologies for delirium in hospitalized COVID-19 patients.
In the present study, we aimed to describe the etiologies, clinical features, and COVID-19 and delirium severities in a prospective series of consecutive delirious COVID-19 patients assessed using standardized tools by a delirium expert liaison psychiatrist working in an ICU. Assessment tools included the Delirium Etiology Checklist (DEC), DSM-5 criteria, Delirium Motor Subtype Scale–4 (DMSS-4), Delirium Diagnostic Tool–Provisional (DDT-Pro), and COVID-19 Clinical Severity Scale (CCSS). Here, we report etiologies for delirium and the relationship between COVID-19 and delirium severities at baseline and follow-up after delirium improvement.
Methods
Design, Eligibility Criteria, Participants, and Ethics
This is a prospective longitudinal study of the first 20 consecutive adult inpatients with SARS-CoV-2 infection (COVID-19 disease) who had delirium diagnosed by the Liaison Psychiatry Service at Clinica Universitaria Bolivariana in Medellín, Colombia (CUB).
SARS-CoV-2 infections were diagnosed in CUB using the Charité/Berlin (World Health Organization) protocol of real-time RNA reverse-transcription into DNA with polymerase chain reaction of E and RdRp genes. According to the laboratory, the protocol sensitivity is 95% when assayed in SARS coronavirus virions, and no false positives in clinical samples pretested positive for other respiratory viruses (
20). According to the CUB protocol, samples for diagnosis were obtained from nasopharyngeal swab in all 20 cases.
Delirium diagnosis was made according to DSM-5 criteria (
21); delirium severity was assessed with the DDT-Pro (
22,
23) and etiologies tabulated with the DEC (
24). COVID-19 severity was measured with the CCSS (
25,
26).
Demographic and medical information were obtained from medical records. The study was approved by our institution’s ethics committee (Comité de Ética de Investigación en Salud de la Universidad Pontificia Bolivariana, Medellín, Colombia), and permission for gathering information from electronic charts was obtained from the CUB research committee.
Instruments
Relevant demographic and clinical variables were collected using a standard method. Clinical variables included medical diagnoses, medications, preexisting dementia diagnosis, and death at follow-up.
Charlson Comorbidity Index–Short Form (CCI-SF).
The CCI-SF quantifies the severity of baseline medical status on a scale from 0 to 1 (no medical comorbidity) to 10 (maximum comorbidity) points, where a score of 2 indicates low comorbidity and ≥3 indicates high comorbidity. CCI-SF assesses eight health conditions; each of the first six adds one point and the last two each add two points. The conditions are stroke, diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure and ischemic heart disease, dementia, peripheral artery disease, chronic kidney failure, and cancer (
27).
CCSS.
The CCSS is an ordinal scale to grade clinical status of COVID-19 patients by the following seven levels: death; hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation; hospitalized, on noninvasive ventilation or high-flow oxygen devices; hospitalized, requiring low-flow supplemental oxygen; hospitalized, not requiring supplemental oxygen, requiring ongoing medical care (COVID-19 related or otherwise); hospitalized, not requiring supplemental oxygen, no longer requires ongoing medical care; not hospitalized (
25,
26).
DDT-Pro.
The DDT-Pro is a three-item brief structured scale for provisional diagnosis and severity of delirium, which can be administered by any health care professional; it has been validated in traumatic brain injury and medical-surgical samples (
22,
23). It contains structured and quantitatively scored items for evaluating vigilance (item 1) and comprehension (item 2) adapted from the Cognitive Test for Delirium (CTD) (
28) that are assessed directly through patient performance without needing verbal responses (useful in mechanically ventilated patients), and sleep-wake cycle disturbance (item 3), a descriptively anchored item from the Delirium Rating Scale–Revised–98 (DRS-R-98) (
29), rated using all sources of clinical information for the preceding 12–24 hours. Item 3 rating values were reversed to align with the scoring for the CTD items. These items represent symptoms from each of the three core domains that are characteristic of delirium (cognitive, higher-level thinking, and circadian) (
22,
23,
30). Total DDT-Pro scores range from 9 points (best) to 0 (worst), where each item is scored 0–3. The best cutoff for diagnosing delirium is a DDT-Pro score ≤6, with scores ranging from 7 to 9 indicating nondelirium (
22,
23). It has two alternate equivalent forms, A and B, for items 1 and 2. The tool was used in this study to quantify delirium severity in patients diagnosed by the liaison psychiatrist.
DEC.
The DEC is a two-part structured tool to determine the attribution of etiologies to an episode of delirium according to 13 categories, including metabolic, infection, primary CNS disorders, systemic disturbances that affect cerebral function, and drug or toxin exposure (including intoxication and withdrawal) (
24). The DEC has a worksheet to capture the specific medical, surgical, and pharmacological conditions that are present and a rating table to systematically rate each etiology category for its degree of likelihood for causing delirium based on overall clinical evaluation of a patient using all sources of laboratory and medical information. The ratings are as follows: definite cause; likely cause; present and possibly contributory; present but apparently not contributory; and ruled out, not present, or not relevant.
Procedures
A liaison psychiatrist assessed, diagnosed, and managed delirium treatment for all SARS-CoV-2 positively tested patients included in this report, usually within a day (range 0–48 hours) after referral by the primary attending physician who suspected delirium. The liaison psychiatrist works closely as part of the team with the attending physicians, who are also trained in clinical delirium detection. Delirium diagnosis was made by the psychiatrist according to DSM-5 criteria (
21), and motor subtypes were determined according to the DMSS-4 as hyperactive, hypoactive, or mixed (
31). The psychiatrist had access to all available information sources and discussed patients’ clinical status with the team; therefore, diagnostic and therapeutic decisions regarding delirium were taken after considering clinical characteristics of each patient.
Preexisting dementia was diagnosed clinically using all available sources (patients, caregivers, and families interviewed by phone if needed; clinical charts; neuroimages). The DEC was completed at baseline visit. The DDT-Pro and CCSS were performed at the first and final psychiatric assessment when delirium had been clinically improved for at least 1 day after stabilization of clinical status, transfer to general ward, or delirium medication down-titration. Patients were followed daily by the liaison psychiatrist for routine delirium management, including psychopharmacological, after the baseline assessment until clinical complications and delirium were resolved.
Statistical Analysis
An SPSS 23.0 database was created. Discrete variables are reported by absolute and relative frequencies (percentages). According to Shapiro-Wilks test, age was normally distributed and is reported with means and standard deviations; the other continuous variables are reported by medians and interquartile ranges. Finally, we performed some exploratory analyses using Spearman’s rho correlations (one-tailed p values) for delirium severity with the DDT-Pro, CCI-SF for baseline medical burden, and CCSS for current COVID-19 status; and mean ranks comparison for baseline DDT-Pro between those who died at follow-up and those alive at last assessment. Significance values were set at p<0.05.
Results
Table 1 shows baseline patient characteristics; 75% were male and age ranged from 50 to 90 years. Prevalence of dementia and preexisting comorbid conditions was low. The CCI-SF score was ≤2 in 19 (95%) patients, without a meaningful correlation with the DDT-Pro (ρ=0.205, p=0.193) or CCSS (ρ=–0.016, p=0.474). Twelve patients (60%) were hypertensive and five (25%) had diabetes.
All or nearly all patients were treated with corticosteroids, anticholinergics, benzodiazepines, and opiates (including fentanyl); half received dexmedetomidine as part of their medical management in the ICU. None received antivirals.
All except two patients were in the ICU during the baseline assessment and on invasive mechanical or high-flow noninvasive ventilation (CCSS scores of 2–4). At baseline, DDT-Pro delirium severity correlated significantly with the severity of COVID-19 on the CCSS (ρ=0.459, p=0.021).
Nearly all patients had a definite likelihood for delirium etiology. The most common definite or likely causes or possibly contributory etiologies were organ insufficiency (100%), systemic infection (100%), and metabolic and endocrine disturbances (95%) (
Table 2). All had between three and four delirium etiologies (from definite to possible contributors); the most common combinations were metabolic and endocrine plus systemic infection plus organ insufficiency (85%) and metabolic and endocrine plus systemic infection plus organ insufficiency plus any other CNS disorder or any other diverse cause (10%). The “any other” CNS etiologies were all preexisting chronic conditions related to cognitive or motor impairment.
According to the DEC worksheet (could have >1 specific condition present in a given category), organ insufficiencies related to delirium were pulmonary (95%), renal (35%), cardiac (10%), and hepatic (5%). Systemic infections related to delirium were viral (85%), followed by a variety of bacterial causes and complications (75%). Metabolic and endocrine etiologies were a variety of acid-base and electrolyte disorders (100%), anemia (60%), hyperglycemia (40%), and hypoalbuminemia (30%).
Delirium characteristics and severity, where 75% of patients had hyperactive motor subtype, are presented in
Table 1. Three (15%) patients had hallucinations; although it was difficult to assess thought content because of oral communication restrictions, one (5%) who did not have hallucinations did have delusions or suspiciousness. The DDT-Pro score range at baseline assessment was 0–6, with eight (40%) patients scoring 0 or 1.
Nonpharmacological therapeutic measures for delirium were applied for all patients, although their isolation needs and biosecurity measures precluded direct family involvement in their care and constricted the interaction between patients and staff. Nonetheless, standard of care included the following: restriction of invasive procedures (central and peripheral lines), hydration assessment, noise control, use of glasses and hearing aids when possible, preservation of environmental cues of day and night as much as possible, reorientation, explanation of procedures, pictures and recorded messages from the family when available, and communication between the staff and the family members and home caregivers.
All patients received antipsychotics as first options for delirium treatment (haloperidol 5–20 mg/day [except 1 mg for patients with hypoactive subtype or no motor subtype], N=16 [80%]; quetiapine 50–600 mg/day, N=4 [20%]). Quetiapine was chosen if there was a history of extrapyramidal adverse events when using typical antipsychotics or for its hypnotic effect administered at night. Three patients had an inadequate response to haloperidol; it was changed to quetiapine in two (12.5%) and levomepromazine 25 mg/day in one (6.2%). Adjunctive trazodone 50–100 mg q.h.s. was added in eight out of 20 (40%) patients for sleep and nocturnal activity regulation.
Median psychiatry follow-up time was 9 days (interquartile range=22) with a range of 3–49.
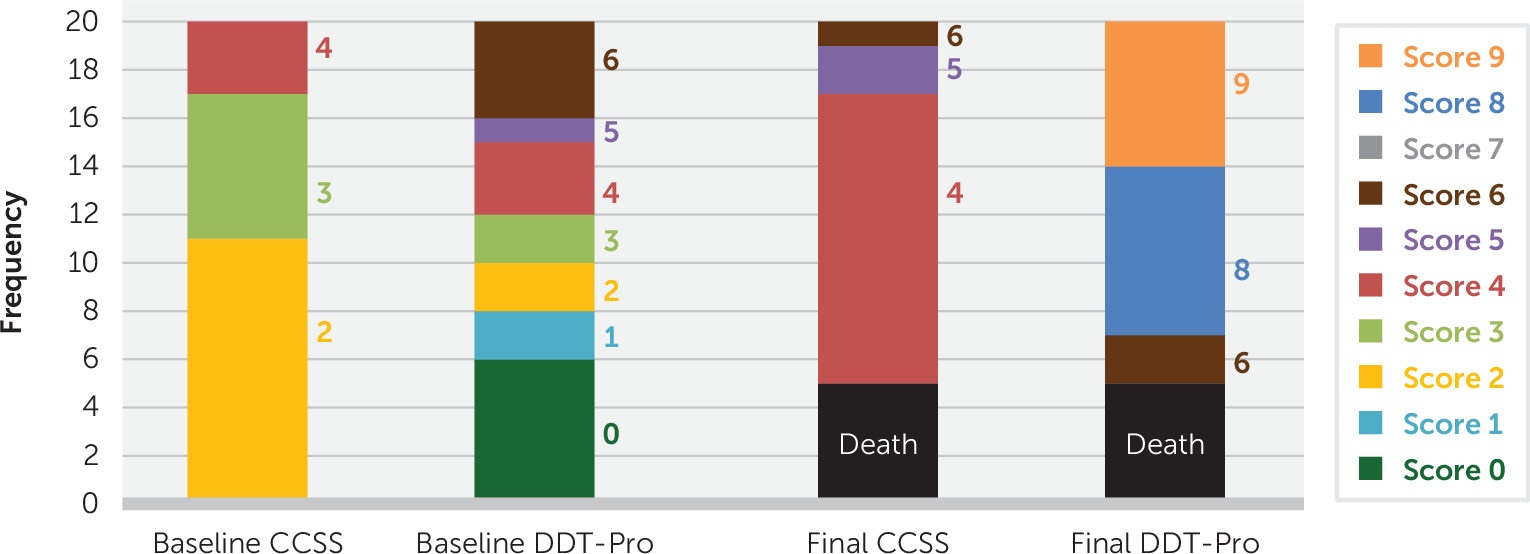
Figure 1 shows rating levels for CCSS and DDT-Pro at baseline (N=20), where CCSS scores are severe stages and DDT-Pro scores indicate severe delirium. At the final assessment (N=15) scores were improved for both scales. Five patients (25%) died before the final DDT-Pro assessment, representing CCSS=1. Final DDT-Pro scores ranged from 6 to 9, and 46.7% of patients received a score of 8. Final CCSS scores correlated significantly with DDT-Pro for survivors (0.567, p=0.028).
The baseline median DDT-Pro score in the 5 patients who died was 1 (interquartile range=4), versus 3 (interquartile range=6) in the 15 survivors (p=0.266). Because the baseline DDT-Pro score in those who died ranged between 0 and 4, we performed a posterior likelihood ratio chi-square test to explore whether the DDT-Pro score ≤4 at baseline was related to mortality (likelihood ratio χ2=3.398; df=1, p=0.065).
Because a p value of 0.065 is close to the significance threshold and death is a critically important outcome, we calculated significance values for the first 10 and then first 15 patients of the study (p=0.201 and p=0.095 respectively), finding a trend toward significance for a relationship with mortality among patients scoring ≤4 on the DDT-Pro as the sample size increased.
No survivor at final assessment required mechanical ventilation or high-flow oxygen support; rather, most were on supplementary low-flow oxygen and hospital medical care (CCSS levels 4 and 5), and all had DDT-Pro scores between 6 and 9. Four of the five patients who died had baseline CCSS=2 (on mechanical ventilation) scores.
Discussion
Given the paucity of prospective delirium studies during the COVID-19 pandemic using standardized tools designed for delirium and administered by delirium expert psychiatrists, we conducted a longitudinal study of 20 inpatients with COVID-19 who met DSM-5 diagnostic criteria for delirium in an academic hospital in Medellín, Colombia, 18 of whom were in an ICU at baseline. These patients were assessed by a liaison psychiatrist expert in both clinical and research delirium evaluation and medication treatment, who managed the patients clinically during the study period until either death or the final evaluation for improved delirium.
Most patients were male, and more than half had chronic hypertension, both recognized risk factors for severe COVID-19 (
7). Surprisingly, baseline preexisting medical problems rated using the CCI-SF did not correlate with delirium or COVID-19 severity. However, there was a correlation between greater severities of COVID-19 (CCSS) and delirium (DDT-Pro) at baseline (p=0.021) and final assessment (p=0.028). Although the CAM-ICU does not measure severity, CAM-ICU delirium in COVID-19 patients with acute respiratory distress syndrome was associated with longer mechanical ventilation time and ICU length of stay (
9). Thus, there appears to be a relationship between delirium and COVID-19 disease burden.
Delirium is common in COVID-19; however, our objective was not to determine incidence but rather to describe the clinical characteristics of delirium and their relationship to etiologies and medical severity. The delirium incidence rate among ICU patients with COVID-19 was reported at 79.5%, based on the CAM-ICU being positive at any time during the hospital stay, and 18% on admission (
9).
Kotfis et al. (
8) described the possible causes for delirium in seven categories: direct CNS invasion, induction of CNS inflammatory mediators, secondary effect of other organ system failure, effect of sedative strategies, prolonged mechanical ventilation time, immobilization, and other needed but unfortunate environmental factors, including social isolation and quarantine without family.
We ascertained etiologies of delirium using the DEC, which attributes the likelihood of pathophysiological causes of delirium using all sources of information from laboratory and medical records, and found that in almost all patients organ insufficiencies (not only pulmonary) and systemic infection were definite causes of delirium; all had at least three other serious medical problems implicated for causing delirium, including metabolic and endocrine disturbances.
Pathophysiological mechanisms for delirium in COVID-19 are many. Inflammatory response to systemic infections alters the blood-brain barrier, allowing a central inflammatory response leading to neuronal dysfunction of cholinergic circuits, among other alterations (
32). Pulmonary, cardiac, and renal insufficiency reduce CNS mitochondrial oxidation, increase CNS dopamine, and decrease acetylcholine production (
33). Hepatic insufficiency increases tyrosine, phenylalanine, and tryptophan levels, leading to increase of CNS dopamine and serotonin (
34). Among metabolic and endocrine perturbations, electrolyte disturbance and anemia alter CNS oxidative metabolism (
35,
36), and hyperglycemia causes direct brain insult and increases neuronal depolarization (
37). The neural circuits altered as a result of these etiologies are related to delirium and involve the reticular activating system and thalamus and their connections to the neocortex, including parietal and frontal lobes (
38,
39).
There were no cases of delirium attributable to direct brain pathology, such as stroke, encephalitis, or meningitis. These seem less common in COVID-19 patients when compared with peripheral pathologies (
3,
9,
10).
Kotfis et al. (
8) recommended that delirium presence should be actively screened for during COVID-19 using dedicated psychometric tools. These include the brief screening tools CAM-ICU and ICSDC used by nurses; delirium severity should be checked using the well-validated Delirium Rating Scale-Revised-98 (DRS-R98) (
8). We used the DDT-Pro, a simple, highly structured three-item tool derived from the DRS-R98 and CTD to assess severity of symptoms representative of the three core domains of delirium. Circadian core domain (sleep-wake cycle) is not assessed by the CAM-ICU. The DDT-Pro is highly correlated with the DRS-R98 score (r=0.913) (
22) and is sensitive to change in delirium severity at follow-up (
23). The DDT-Pro may be administered by any clinical staff member for delirium diagnosis and severity; its cutoff score ≤6 has 88%–90% sensitivity and 85%–87% specificity for an independent DSM-5 delirium diagnosis when administered by a physician or nurse to geriatric inpatients (
23).
DDT-Pro score did not correlate with baseline medical burden (CCI-SF) in our sample, where almost all patients had ≤2 premorbid conditions and low dementia prevalence. However, according to our exploratory analysis, staff treating more severe COVID-19 patients should anticipate more severe delirium (
Figure 1), even in patients with a low number of preexisting medical comorbidities. Although nonsignificant, the DDT-Pro score at baseline among the five (25%) patients who died was lower than that of the 15 (75%) who survived, and there was a trend for those scoring ≤4 to have increased frequency of death during the follow-up, suggesting a predictive relationship for mortality with more severe delirium in COVID-19.
According to the DMSS-4, 80% of our patients had hyperactive subtype or mixed motor subtype delirium, which is consistent with the reported high prevalence of the Richmond Agitation–Sedation Scale +3 and +4 scores (very agitated and combative) (
9). We found that at least 20% of the sample had hallucinations or delusions and suspiciousness. These findings indicate that patients with COVID-19 are more disruptive, cause more stress among staff members, and need more pharmacological interventions. The use of nonpharmacological measures for delirium management is possible but restricted due to patient biosafety isolation needs and limitations on staff availability.
Well-designed clinical trials of delirium treatment for COVID-19 patients are lacking, and our study is not a clinical trial; rather, it is a descriptive study of routine clinical care. The use of antipsychotics for reducing delirium severity or incidence is common in clinical practice by psychiatrists, and they are generally well tolerated, as supported by clinical trials (
40–42). Additionally, metanalysis supports the efficacy and safety of haloperidol (as the first pharmacological option) and quetiapine, the two agents most frequently used in our sample (
43). Decades of experience also support the safe use of antipsychotics in the ICU, and their safety was corroborated by a recent systematic review (
44). Recommendations based on case reports of COVID-19 patients indicate the use of antipsychotics for delirium treatment as a result of their blocking effect of dopamine excess and rebalancing of the cholinergic deficiency in delirium, such as for thalamic sensorimotor gating (
12,
24,
33,
38).
Although all patients in our cohort received antipsychotics for delirium, almost half required the addition of trazodone for sedation; 15% needed an antipsychotic change to a more sedating option due to insufficient response to haloperidol. Delirium pharmacological management in the ICU is complicated by the deliriogenic effects of intoxication or emergence withdrawal from sedation and pain medications commonly used in the ICU setting (
45,
46). Nearly all of our patients received drugs known to be deliriogenic as part of their ICU treatment regimen–corticosteroids, anticholinergics, benzodiazepines and opiates–suggesting that COVID-19 conditions were not the only etiologies for delirium. Interestingly, none of these drugs was considered a main cause of delirium on the DEC, perhaps because they were a standard of care and overshadowed by major physiological impairments. Only half received dexmedetomidine for sedation, reported to sedate with less delirium risk (
18,
19,
47).
Melatonin has been reported to help maintain a normal sleep-wake rhythm in COVID-19 delirium and has possible anti-inflammatory and immune-enhancing features (
48). A clinical trial using ramelteon, a melatonin receptor agonist, supported a pharmacological intervention in the circadian system for delirium prophylaxis (
49). Circadian abnormalities are one of the three core symptom domains of delirium. Unfortunately, neither of these two medications are available for routine hospital use in Colombia.
Our study is limited by a small number of consecutive cases, but its strengths were the use of valid delirium-specific tools by an expert liaison psychiatrist and longitudinal follow-up. The DDT-Pro scores correlated to COVID severity at both baseline and final assessments. Although studies in larger samples are needed, more severe delirium on admission to ICU for COVID-19 may be a harbinger of mortality even in patients who did not have much preexisting medical comorbidity on the CCI-SF. Future research with larger samples using multivariate analyses is needed to study relationships of concomitant deliriogenic medications and key medical etiologies for delirium such as organ insufficiency with severity of delirium in COVID-19 patients. Patients with COVID-19 should be assessed for delirium severity.