In 1925, the first morphological descriptions of a distinctive group of nerve cells, von Economo neurons (VENs), were published in an epic atlas depicting the cortical organization of the human brain (
2). The book containing these descriptions was written by the Australian neurologist Constantin Alexander von Economo (1876–1931) and his colleague George Koskinas (1885–1975) (
17). von Economo named these neurons “rod and corkscrew cells,” denoting their straight and twisted morphological variations (
2,
4). VENs were described as large and spindle-shaped bipolar neurons, localized primarily within frontolimbic regions. Over the past century, VENs have been identified as specialized cells in humans and in other primates in the hominid lineage (e.g., bonobos, common chimpanzees, orangutans, and gorillas) but not nonhominid primates (e.g., particular monkeys and apes) and nonprimate mammals (e.g., crows and pigs) (
3–
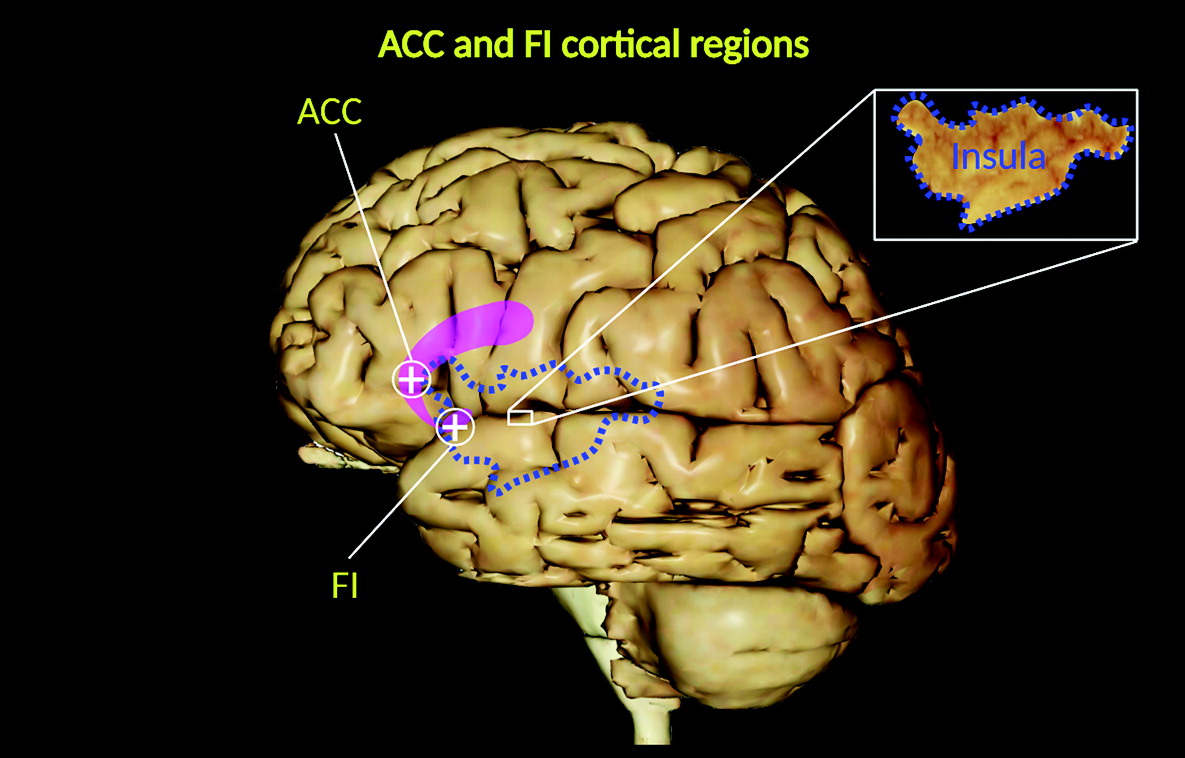
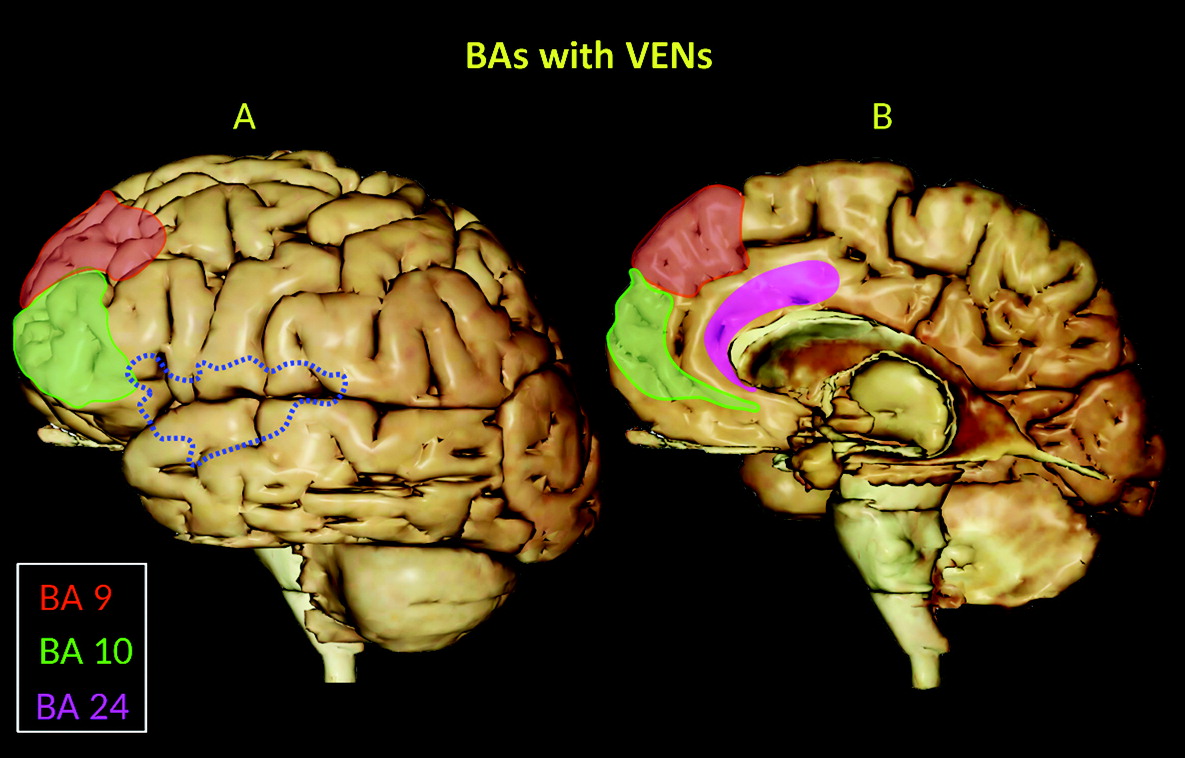
5). They are located in the deep portion of cortical layer V (sublayer Vb), as well as in the frontoinsular (FI) cortex, prefrontal cortex, and anterior cingulate cortex (ACC) (
3–
5) (
Figure 1), and they are widely distributed in the temporal lobe, including the entorhinal cortex, subiculum, and hippocampal formation (
1,
11–
13,
15) (
Figures 2 and
3).
Of these regions, the FI cortex and ACC represent key functional centers within a wide neural network (salience network) involved in social–emotional, homeostatic, and autonomic responses (
7–
10). Clinical neuroscientists have been increasingly interested in VENS because of their possible connection with mental disorders, especially those affecting social skills (i.e., social awareness, social judgment, and social humor), self-control, empathy, and self-perceived life satisfaction (
1,
5,
18,
19).
Neurobiological Profiles
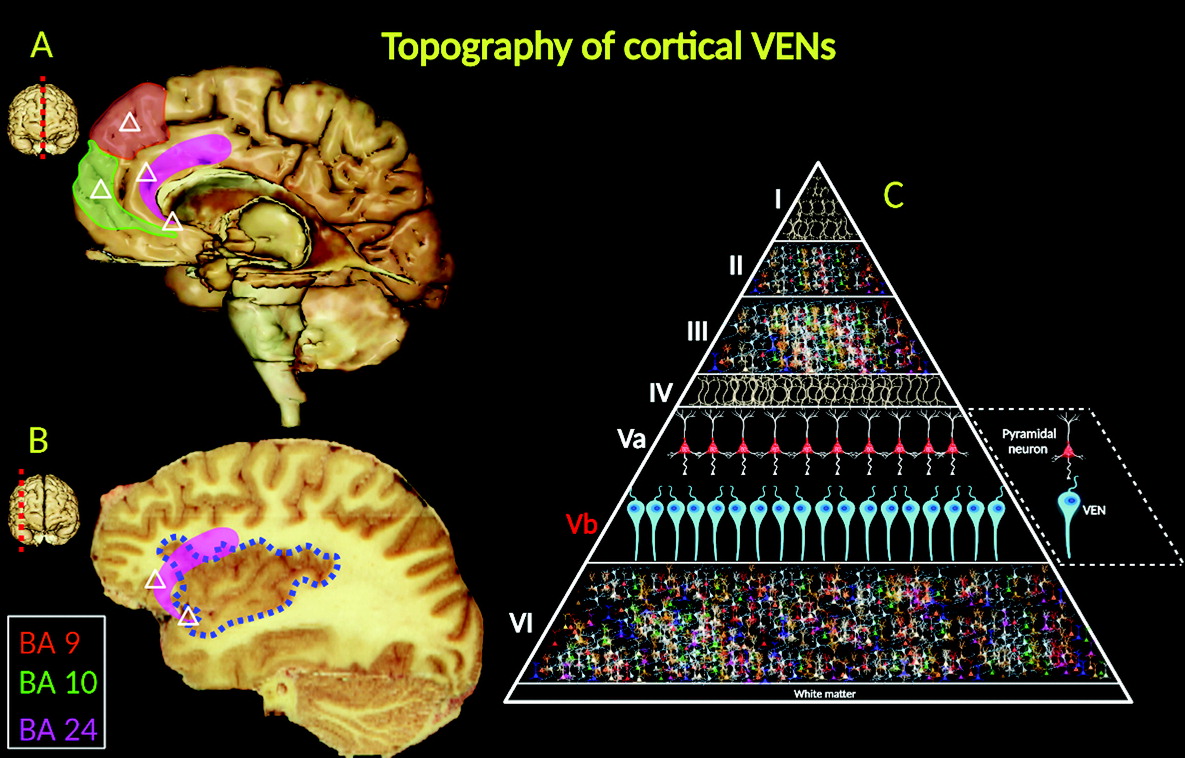
VENs are projection neurons, typically exhibiting larger cylindrical soma than pyramidal nerve cells (
15). Morphologically, VENs differ from pyramidal cells in that they have a singular large basal dendrite; by contrast, pyramidal neurons have smaller dendrites. VENs possess a distinctive, narrow dendritic arborization pattern, spanning into the laminated architecture of the cerebral cortex and possibly allowing faster relay of neuronal signals (
16) (
Figure 3C).
Another distinctive feature of VENs is their structural symmetry. One study suggests that there is a high level of symmetry between the apical and basal dendrites of VENs (
16). Other investigators have described apical and basal dendritic processes that appear to be perpendicular extensions toward the pial surface and the subcortical white matter, respectively (
11,
13). A recent morphological study reported that the basal part of the body axis of VENs is distinctive, exhibiting a uniquely spiral and relatively thick origin of the apical stem followed by a gradual thinning. Perhaps the most peculiar characteristic of VENs is their axon, originating from the ending of the basal extension and occasionally sharing a common origin with a dendrite (
20).
The cellular density distribution of VENS in humans seems to vary based on the cortical regions where VENs reside (
6). Interestingly, the density of VENs appears to decrease from the ACC and insula toward the frontal surface and the superior frontal gyrus (
1). Other studies have examined the embryological development of VENs in the ACC and FI cortex in humans. While neurogenesis of cortical layer V pyramidal neurons occurs approximately between gestational weeks 6.5 and 10, VENs first appear in week 36 postconception and are limited in number at birth (
6). However, the number of VENs in the human brain increases significantly during the first 8 months after birth and postnatal asymmetries in VEN numbers develop, with more VENs in the right than the left FI cortex and limbic anterior region of the ACC (
6).
VENs express high levels of nonphosphorylated neurofilament protein, as well as other important receptors, including vasopressin (1A), dopamine (D
3), serotonin (2B), and gamma-aminobutyric acid (GABA) subunit theta (
GABRQ) receptors (
21–
23). VENs, as well as fork cells, are intensely labeled by the bombesin peptides neuromedin B (NMB) and gastrin-releasing peptide (
1). These peptides have peripheral roles regulating the release of digestive enzymes, peristaltic contractions, and immunological responses to the ingestion of toxic agents. In the insula, labeling by these peptides suggests that VENs may be involved in the neurophysiological integration of visceral interoceptive mechanisms potentiated by higher brain functions (i.e., motivation and decision making) (
21,
24).
Several studies have investigated the expression of transcription factors in VENs. One study reported that VENs express the transcription factors FEZF2 and CTIP2, which regulate the developmental endpoint and differentiation of projection neurons toward that end. This finding suggests that VENs provide a potential link between certain autonomic control sites in the cortex (i.e., layer V of the FI cortex and ACC) and the brainstem or spinal cord regions (
25). Another study analyzed the expression of the protein cyclic adenosine monophosphate–dependent activating transcription factor 3 (ATF3), as well as the expression of other proteins (interleukin-4 receptor alpha and NMB), in the ACC (
26). In nonhuman animals, the function of ATF3 includes the regulation of pain in the spinal cord and modulatory stress responses from physiological stressors, such as liver trauma, myocardial ischemia, and seizures (
27). Thus, the expression of ATF3 in VENs in the ACC suggests a possible modulatory role of these neurons in pain perception (
26).
Neuropsychiatric Implications
A growing body of evidence implicates the disrupted-in-schizophrenia 1 protein (DISC1; a product of the
DISC1 gene) as a genetic susceptibility factor for schizophrenia (
28–
32). DISC1 was initially discovered and linked to a translocation in chromosome 1 through four generations of a large Scottish family and cosegregated with a spectrum of mental illnesses, including not only schizophrenia but also schizoaffective disorder, recurrent major depression, and adolescent conduct and emotional disorders (
33). Another study reported similar genetic mutations at the DISC1 carboxyl-terminus in a smaller American family with a history of mental illnesses and suggested that
DISC1 gene mutations could increase the risk for schizophrenia and schizoaffective disorder (
34). This gene is also associated with cell proliferation, differentiation, and migration regulatory processes, as well as with the organization of dendritic architecture (
1,
25,
35). DISC1 protein is expressed in the soma and dendritic processes of VENs (
6). Accordingly, DISC1 protein involvement in the regulation of dendritic structure may affect VEN morphology and cytoarchitecture organization (
1,
6,
25) and, by extension, suggests a role for DISC1-related alterations of VENs in schizophrenia spectrum disorders and related psychiatric disorders.
Individuals with early-onset schizophrenia have been shown to have lower VEN density in the ACC than individuals with bipolar disorder and individuals without psychiatric diagnoses (
36). Age, sex, postmortem interval, brain weight, and cortical thickness appear to have no significant impact on the reduction in VEN density in the ACC in individuals with early-onset schizophrenia (
36). Ultrastructural alterations in ACC VENs, including an increased number of lysosomal aggregations, have also been observed among persons with schizophrenia. VENs in limbic structures (i.e., the ACC and FI cortex) may be neurodevelopmentally altered in schizophrenia and possibly associated with suicide among persons with psychotic disorders (
36–
38). Furthermore, these pathological changes possibly explain the changes in morphology and connectivity of the ACC seen among patients at risk for developing psychosis (i.e., psychosis in 22q11.2 deletion syndrome) (
39).
Another neuropsychiatric condition in which the alteration or loss of VENs may be clinically relevant is frontotemporal dementia (FTD). In this neurodegenerative disorder, social behavior and emotions are characteristically affected, particularly in the behavioral variant of FTD (bvFTD) (
40,
41). Seeley et al. (
41) noted that FTD is associated with early, severe, and selective VEN losses (up to 74%) in the ACC-FI network. They further noted that similar VEN reductions were not observed in Alzheimer’s disease, even in its late stages. Stereological studies have indicated that VENs are selectively targeted in the early stages of bvFTD and appear to be related to impairments in empathy, self-control, and social awareness (
42,
43). Other investigations have supported the idea that VENs are more susceptible to loss in persons with FTD than in a control group and in patients with Alzheimer’s disease (
44,
45). Accordingly, VEN pathobiology appears to contribute to social-emotional deficits in persons with FTD (
46), including empathy deficits among individuals with bvFTD (
41).
The role of VENs among persons with bvFTD with a C9orf72 expansion also merits consideration. Persons with C9orf72 bvFTD have early social, emotional, and cognitive impairments that have been linked to alterations in VENs (
47). A reduction in the number of VENs, including VENs expressing GABRQ in the ACC, was observed in C9orf72 bvFTD (
47). The GABRQ is expressed on a monoaminergic phenotype of VEN, and its selective vulnerability in C9orf72 bvFTD was associated with impairments in social cognition but not psychotic symptoms, memory or speech impairments, or motor neuron disease (
47). This suggests that this subgroup of VENs is a key modulator of social and emotional functioning and that its alteration is an important contributor to the clinical phenotype of C9orf72 bvFTD; by extension, these neurons may be similarly critical to disturbances in this domain of neuropsychiatric function in other disorders (
47).
Among the other neuropsychiatric disorders linked to alterations in the number, structure, and location of VENs are autism spectrum disorders (
1). Social cognitive impairments are key features of this class of disorders (
40). Allman et al. (
21) reported that the social disabilities observed in those with autism spectrum disorders may be related, in part, to abnormal VEN development. Additionally, reductions in VENs in the anterior insula also may result from alcohol dependence (
48), possibly reflecting the sensitivity of the
GABRQ VEN subgroup to GABA receptor stimulation by alcohol (
49). Given the effects of chronic alcohol use disorder on socioemotional function and social cognition, the role of VENs in the development of these and related adverse outcomes from alcohol use disorder warrants further study (
50).
Conclusions
VENs have a unique anatomical distribution within limbic system structures of the human brain. In particular, the ACC and FI cortex possess a high population of VENs. Although the specific function of VENs remains unknown, their exclusive topographic location within the FI cortex and ACC suggests a potential role in socioemotional function, including social cognition, and related autonomic functions. Given the role of VENs in these aspects of neuropsychiatric function, the findings linking alterations in the number, structure, or function of VENs in neuropsychiatric disorders, such as schizophrenia spectrum disorders, bvFTD, autism, and alcohol use disorders, are understandable. VENs perform novel and nonclassical cortical modulatory functions in the FI cortex and ACC, which distinguish them from most neurons within limbic, paralimbic, and prefrontal cortices. Further research is necessary to refine the function of VENs and to identify their relevance as potential therapeutic targets for the neuropsychiatric conditions in which they feature prominently.