Case Vignette
A 66-year-old right-handed male with a 5-year history of akinetic-rigid Parkinson’s disease is referred for neuropsychiatric consultation to evaluate nightmare symptoms and possible posttraumatic stress disorder (PTSD). He is accompanied by his wife, who describes episodes of screaming and yelling while the patient is asleep that began nearly 10 years ago; 2 years later, he started “acting out his dreams.” In a typical episode, according to his wife, the patient appears to run away from someone, punches the air, and repeatedly yells, “get away from me.” The episodes occur 2–3 hours after he falls asleep, four to five times per week. He does not recall the dreams or episodes. His wife denies that he snores or stops breathing in his sleep. She also denies that he has ever walked out of bed or injured himself during an episode, but she sleeps in a separate bed for her own safety and sleep quality. The patient denies excessive daytime sleepiness, sleep paralysis, sudden losses of muscle tone, or hallucinations upon waking or falling asleep.
The patient assumes that his nocturnal episodes stem from him being sexually assaulted in his early twenties. Since that assault, he has reported the experience of intrusive memories and fear, as well as hyperarousal, when reminded of the event. Additionally, he avoids situations that remind him of the assault, tends to mistrust and detach from others, and demonstrates hypervigilance, an exaggerated startle response, and easy irritability. He denies the experience of discrete nightmares that he can recall upon waking. In recent years, he began to discuss his sexually traumatic event with his wife, with the hope it would improve the dream enactment behaviors.
The patient denies a past history of traumatic brain injury or epilepsy. His current medications include carbidopa/levodopa (25/100 mg, two tablets alternating with 2.5 tablets every 2 hours from 8:00 a.m. to 8:00 p.m.); fluoxetine (40 mg daily); clonazepam (0.5 mg at bedtime); and prazosin (1 mg at bedtime). Fluoxetine and prazosin were prescribed by a psychiatrist for PTSD symptoms and nightmares, respectively. A neurologist prescribed clonazepam for dream enactment behaviors and suspected REM sleep behavior disorder (RBD). Prior to this visit, the patient never had a sleep study.
Diagnostic polysomnography is ordered to clarify the diagnosis. Total time asleep is 333 minutes with 73% sleep efficiency. Total time in sleep stages includes 7.1% in stage N1, 70% in stage N2, 22.9% in stage N3, and 0% in REM. His respiratory disturbance index is 0, and his apnea-hypopnea index is 0.3. There are no periodic limb movements and no dream enactment events. Because the patient does not achieve REM sleep during this sleep study, polysomnographic confirmation of RBD is not possible.
Following the sleep study, the patient continues to report dream enactment behaviors. In response, the sleep medicine clinician independently adds melatonin, titrating to 5 mg nightly, and increases clonazepam to 1.5 mg at bedtime for presumed RBD. However, the nocturnal events decrease only to two or three times per week. As a result, the psychiatrist increases prazosin to 2 mg at bedtime for nightmares. Two separate attempts at PTSD-focused psychotherapy are thwarted by exacerbations of PTSD symptoms and cognitive decline. A repeat polysomnogram is recommended by sleep medicine but not completed. The patient remains on clonazepam for the dream enactment behaviors, and his cognition and physical status continue to decline.
Introduction
PTSD is experienced by an estimated 8.3% of the U.S. general population, 10.2% of men and 15.5% of women seeking health care from the Veterans Health Administration, and 23% of veterans of Operation Enduring Freedom/Operation Iraqi Freedom (
1–
3). Referred to as a hallmark of the disorder, sleep disturbances in PTSD are nearly ubiquitous (
4). The contribution of sleep disturbances to PTSD severity, treatment resistance, and even suicide risk emphasizes the importance of identifying and addressing the nocturnal phenomena of PTSD (
5,
6).
The criteria for PTSD in the DSM-5 (
7) include, among other symptoms, insomnia (difficulty falling or staying asleep) and nightmares (repeated, disturbing dreams of the traumatic experience). However, some disruptive nocturnal phenomena, namely dream-enactment behaviors (e.g., yelling, screaming, kicking, and thrashing in response to dream content), are not accounted for in the sleep-related DSM-5 criteria for PTSD (
8,
9).
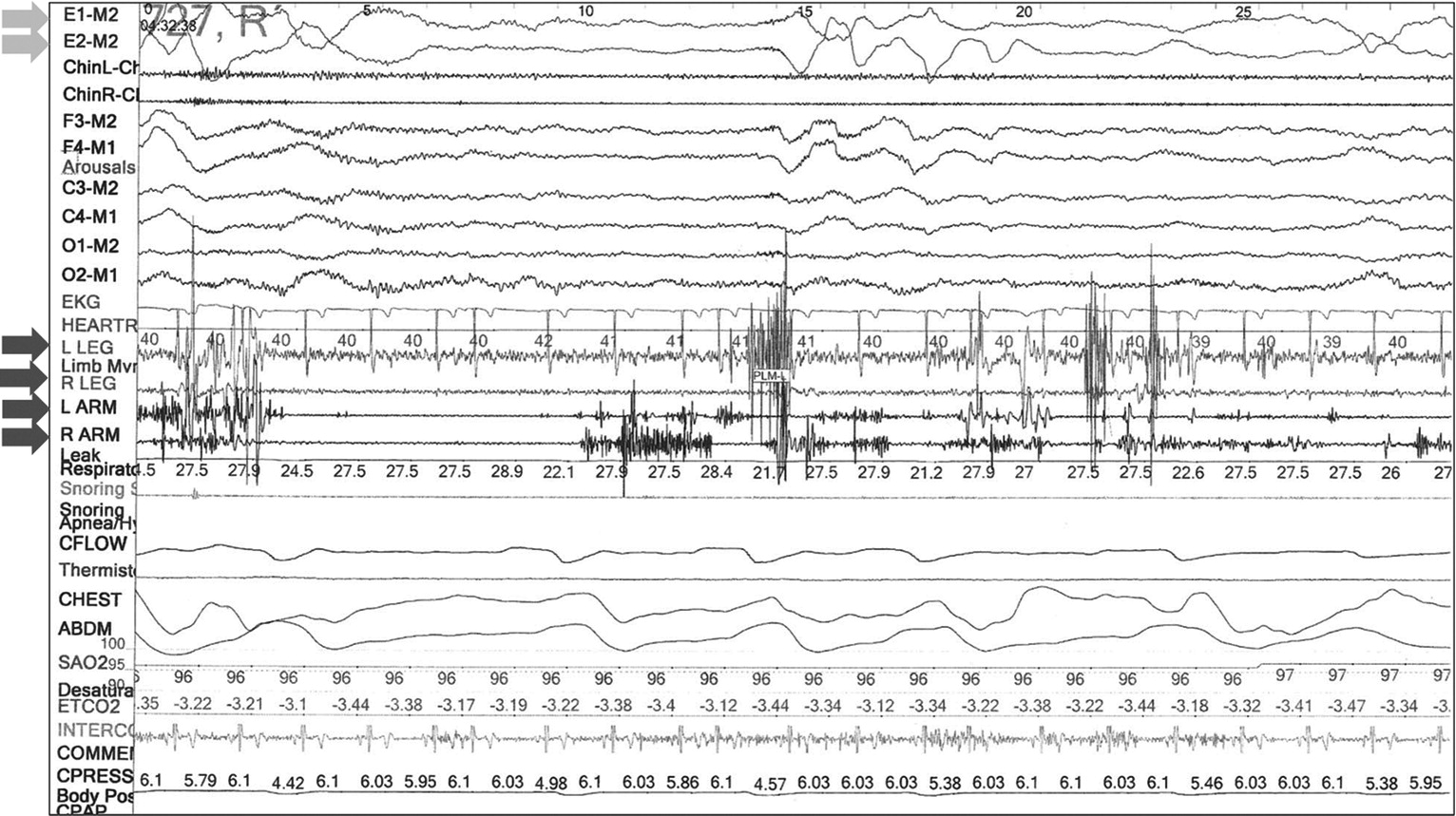
Although common in patients with PTSD, these dream enactment behaviors share phenomenological similarities to other parasomnias. Importantly, they are the defining clinical feature of REM sleep behavior disorder (RBD), which is characterized by loss of the typical atonia of REM sleep, or REM sleep without atonia (RSWA) (
Figure 1). The diagnosis of RBD requires that a patient or their bed partner report dream enactment behaviors and demonstration of RSWA on attended polysomnography (
10). Given that presence of RBD often heralds or manifests underlying neurodegenerative disease, most commonly a synucleinopathy such as Parkinson’s disease (
10), its consideration in the differential diagnosis of PTSD-related sleep disturbances is critical for appropriate management.
A recently proposed parasomnia, trauma associated sleep disorder (TASD), may also present with dream enactment behaviors. This diagnosis incorporates elements of PTSD and RBD, including the incitement of symptoms by exposure to emotional trauma and elevated RSWA, respectively (
11). However, TASD falls short of characterizing a significant proportion of patients with PTSD and RBD (
12,
13). Dream enactment behaviors also occur in conjunction with other sleep disorders, including non-REM parasomnias (e.g., somnambulism), nocturnal epilepsy, obstructive sleep apnea, restless leg syndrome, or overlap parasomnias (
14).
Clinically, the disjointed nosology, which also spans disciplines, translates into unclear treatment guidelines for patients with PTSD and dream enactment behaviors. Furthermore, as illustrated in the preceding case vignette, treatments initiated for PTSD or RBD can confound accurate diagnosis of the etiology (or categorical diagnosis) of these sleep-related behaviors.
This article highlights the clinical need for improved diagnostic and treatment algorithms for dream enactment behaviors associated with PTSD. After defining dream enactment behaviors, the overlapping clinical phenomena of PTSD-nocturnal behaviors, RBD, TASD, and other sleep disorders are described. The role of attended polysomnography and coordination among psychiatry, neurology, and sleep medicine disciplines is emphasized as a necessary element of the diagnostic approach to these conditions. Thereafter, the need for research incorporating polysomnography and longitudinal study of the potential relationships between dream enactment behaviors, PTSD, RBD, and neurodegeneration risk is highlighted.
Definition of Dream Enactment Behaviors
The strictest definition of dream enactment behavior requires capture of the relevant sleep-related behaviors via video recording during overnight polysomnography and, immediately after the recording, confirmation from the patient that the movements corresponded to the dream content (
15). However, clinicians and researchers rarely perform this level of assessment. More commonly, clinical assessments for presence of dream enactment behaviors are retrospective and based on asking “Do you ever act out your dreams while you are asleep?” This question does not distinguish whether the patient or bed partner assumes that the behaviors are related to dreams, if they are a reinterpretation of other sleep-related phenomena (e.g., arousals from obstructive sleep apnea), or if movements during sleep are influencing dream content (
16).
Standardized questionnaires parallel this method of retrospective inquiry. For instance, the Single-Question Screen for RBD asks, “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep (for example, punching, flailing your arms in the air, making running movements, etc.)?” (
17). Similarly, item 1g of the Pittsburgh Sleep Quality Index Addendum for PTSD asks participants whether they have had episodes of “acting out” dreams, such as kicking, punching, running, or screaming (
8).
For present purposes, then, dream enactment behaviors are defined as self- or bed-partner-reported acting out of dreams, whether or not they are captured on video or definitively attributed to dream content. This definition aligns with standard clinical and research inquiries about the presence and frequency of dream enactment behaviors.
Etiological Considerations
As illustrated in the case vignette, presentation and management of dream enactment behaviors span the scope of practices of sleep medicine, psychiatry, and neurology. Accordingly, assumptions of causality and treatment of nocturnal symptoms in PTSD can be influenced by the perspectives of the clinician’s specialty. The potential pitfalls of uncoordinated multidisciplinary care can be avoided by a framework that considers dream enactment behaviors in patients with PTSD within the context of the broader differential diagnosis of etiologies and conditions that mimic RBD.
Trauma Exposure
The experience of a traumatic event, either personally or as a witness, is the sine qua non for a diagnosis of PTSD. At issue is whether sleep disturbances such as nightmares or insomnia in a person with PTSD are a function of trauma-related emotional arousal or are independent conditions. Currently, clinical features emerging after the traumatic event that support a diagnosis of PTSD, as per the DSM-5, comprise four main symptom clusters: re-experiencing the trauma, avoidance of stimuli akin to the original trauma, negative changes in mood and cognition, and increased arousal (
7). Nightmares, defined as recurrent and distressing dreams, are cited as an example of re-experiencing a trauma. Insomnia, defined as difficulty falling or staying asleep, is regarded as a sign of heightened arousal. When restricted to the DSM-5 nosology, the implicit assumption is that the trauma, and emotional trauma specifically, induces the sleep disturbance.
Other studies describe dream enactment behaviors as a consequence of PTSD-related symptoms. To that end, Germain et al. (
8) developed the Pittsburgh Sleep Quality Index Addendum for PTSD to capture the presence and frequency of disruptive nocturnal behaviors frequently reported in patients with PTSD. Validation studies of this questionnaire demonstrate a correlation between dream enactment behavior symptoms and PTSD severity (
8,
9).
Others speculate that PTSD is characterized by disrupted REM sleep, including dysregulation of mechanisms that normally regulate REM atonia. For instance, Ross et al. (
18) reported increased percentage of anterior tibialis limb twitch bursts in 12 Vietnam veterans with PTSD compared with 10 healthy veteran control subjects. Mysliwiec et al. (
19) also reported a case series of four active-duty service members with disruptive nocturnal behaviors and elevated muscle tone during REM sleep.
TASD has been proposed as a new parasomnia characterized by a history of inciting emotional trauma, RSWA, and disruptive nocturnal behaviors (
11,
19). Nightmares in TASD may occur during REM or non-REM sleep, and patients may or may not meet full criteria for PTSD. TASD is further distinguished from RBD by presence of sympathetic hyperactivity and onset within proximity to the trauma, neither of which are typical for RBD.
Whereas the constellation of symptoms in TASD is attributed to limbic dysregulation during sleep (
11), the links between emotional trauma exposure and dysregulated REM muscle atonia are unclear. The case vignette at the start of this article describes a not uncommon clinical presentation that challenges the thesis that dream enactment behaviors in a patient with Parkinson’s disease can be attributed clearly to remote emotional trauma and TASD rather than to a co-occurring synucleinopathy.
REM Sleep Behavior Disorder
The possibility that PTSD symptoms and dream enactment behaviors are linked to RBD via a shared potential for neurodegeneration is a matter of debate, as PTSD itself is associated with abnormalities of REM sleep. Husain et al. (
20) raised the possibility of shared pathophysiology between PTSD and RBD based on a retrospective chart review of 27 veterans with RBD, in which 56% had concurrent PTSD. Traumatic brain injury (TBI) comorbid with PTSD represents another confounding condition in relation to etiological attribution of RBD, as TBI confers a greater risk of RBD and Parkinson’s disease than PTSD alone (
21,
22). Further, while RBD was historically thought to occur in patients without psychiatric comorbidity, evidence suggests this is a false dichotomy; indeed, the term “psychiatric RBD” (i.e., RBD occurring in patients with psychiatric disorders, including PTSD) has been suggested as a term to capture the occurrence of RBD among persons with such conditions (
23).
To investigate relationships between PTSD, RBD, and TBI, Elliot et al. (
12) used clinical data from 394 veterans consecutively referred for in-lab video polysomnography. Even after excluding participants with concurrent antidepressant use, which was regarded as a possible etiologic factor in RBD, prevalence of RBD was 9% in the overall sample, 15% in the PTSD group (with an age-adjusted prevalence odds ratio=2.81), and 21% in participants with both PTSD and TBI (age-adjusted prevalence odds ratio=3.43). However, no participants met criteria for TASD, defined as the presence of dream enactment behaviors and RSWA (i.e., RBD); an inciting traumatic experience; dream mentation related to the prior traumatic experience; and evidence of autonomic hyperarousal not due to sleep-disordered breathing. Whereas nine of the 34 participants had TBI or PTSD (proxies of exposure to an inciting traumatic event) and dream mentation related to the prior traumatic experience, none demonstrated autonomic nervous system hyperarousal concurrently with abnormal REM muscle activity, excluding those epochs that co-occurred with an apnea or hypopnea (
12).
A retrospective study from South Korea compared frequency of RBD between 20 patients with PTSD, 23 exposed to trauma without PTSD, and 21 without trauma or PTSD (
24). Stepwise logistic regression analyses indicated that PTSD was a stronger predictor of RBD compared with the trauma-exposed group without PTSD after controlling for age and apnea hypopnea index. In light of the small sample size, the authors noted a tenuous link between trauma exposure and development of enactment behaviors, as trauma exposure occurred over 40 years prior, long before development of sleep problems requiring a sleep study referral (
24).
Validation of a relationship between PTSD and RBD requires larger prospective cohort studies with more representative samples (e.g., including nonveterans and women) using standardized sleep, psychiatric, neurological, and polysomnographic assessments. In patients with known neurodegenerative diseases, more research is needed to characterize the impact of PTSD on RBD symptoms. For now, RBD must, at a minimum, be considered in the differential diagnosis of PTSD-related dream enactment behaviors.
Symptomatic REM Sleep Behavior Disorder
Discussion of the differential diagnosis of PTSD-related dream enactment behaviors requires brief mention of several conditions that may manifest or elicit RBD. Narcolepsy, the most common cause of RBD in patients younger than 50, should be considered in the context of excessive daytime sleepiness, hypnopompic or hypnogogic hallucinations, sleep paralysis, or cataplexy (
25). Causes of “lesional RBD” include vascular, neoplastic, demyelinating, and other autoimmune pathologies injuring the dorsal pons or projections of the subcoeruleus. RBD may also manifest with Wilson’s disease, spinocerebellar ataxia type 3, autoimmune encephalitides (e.g., anto-IgLON-5, contactin-associated protein-like 2, leucine-rich glioma-inactivated 1 antibody syndromes), beta-blocker exposure, and alcohol withdrawal, among other conditions (
25–
28).
Of relevance is the potential for antidepressants to induce or unmask RBD. Implicated antidepressants include selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors, mirtazapine, tricyclics, and monoamine oxidase inhibitors (
27). While exact mechanisms are unclear, antidepressants are thought to induce RSWA, ultimately causing dream enactment behaviors and fully developed RBD. Co-occurrence of prodromal features of synucleinopathies with antidepressant-induced RBD implies these medications may be unmasking early alpha-synuclein pathology in susceptible individuals (
29). These longitudinal relationships between neurodegeneration and antidepressant-induced RBD require further investigation (
30).
Other Sleep Disorders
A source of additional confusion lies in the association of dream enactment behaviors with several other sleep disorders besides RBD, TASD, and PTSD-associated nocturnal symptoms. For instance, nocturnal epilepsy, non-REM parasomnias, severe obstructive sleep apnea, and periodic limb movement disorder can be associated with dream enactment behaviors (
28). Dream enactment behaviors are also reported to occur in healthy individuals (
14). Finally, overlap parasomnias or more than one diagnosis (e.g., nocturnal epilepsy or obstructive sleep apnea co-occurring with RBD) are possible.
Dream enactment behaviors associated with obstructive sleep apnea or periodic limb movement disorder may abate when these underlying disorders are treated. Nocturnal epilepsy is differentiated by its stereotyped nature and relative lack of associated dream mentation. Non-REM parasomnias tend to manifest in the first half of the night and are associated with amnesia for the event. Importantly, nightmares, nocturnal panic attacks, non-REM parasomnias, and other causes of dream enactment behavior should not be associated with RSWA, at least when occurring in isolation from RBD (
31).
Implications for Clinical Assessment
When evaluating a patient with PTSD and dream enactment behaviors, clinicians may refer to current practices for parasomnia assessment, again with an emphasis on assessing for conditions mimicking or manifesting with RBD. History-taking includes a detailed description of the behavioral events (preferably from a witness); duration, timing, and frequency of the behaviors; presence or absence of event recall; a family history of similar events; potential aggravating or alleviating factors; and the presence of sleep-related injury to the patient or bedpartner (
25,
28).
Additional inquiry into symptoms of early synucleinopathies (e.g., tremor, constipation, orthostasis, falls), narcolepsy, epilepsy, and obstructive sleep apnea may identify conditions manifesting with RBD or a RBD mimic. Substance- and medication-related triggers of RBD, including alcohol withdrawal, antidepressant use, and beta blockers, should be identified and addressed, if possible. However, the risks and benefits of tapering or replacing medications must be considered in the context of the patient’s comorbidities and preferences. Furthermore, the association of RBD with synucleinopathies, focal lesions, autoimmune encephalitides, Wilson’s disease, and spinocerebellar ataxia type 3, imply the utility of neurological and cognitive examinations and, if indicated, further neuroimaging and laboratory investigations (
25,
28).
In-laboratory polysomnography incorporating video and, ideally, upper-extremity electromyogram (EMG) are currently the gold standard diagnostic tools for parasomnias, as they allow for characterization of sleep stages, possible epileptiform abnormalities, and sleep-related movements (
14). Finally, in patients with suspected narcolepsy, a multiple sleep latency test may aid in diagnosis (
25,
28).
Specifically, for the diagnosis of RBD, the optimal duration and number of nights of video polysomnography is uncertain. Some experts recommend a minimum REM duration of 10% of total sleep time, but at least 5 minutes of REM sleep may be acceptable. Because muscle activity (especially tonic chin activity) during REM sleep is correlated across consecutive nights, one night of recording is generally sufficient (
27).
Treatment Approaches
Treatments for PTSD-associated dream enactment behaviors are directed toward the underlying cause and any associated conditions. The sections below focus on current recommendations for the treatment of PTSD, RBD, and TASD, as well as the potential pitfalls of these recommendations. An overview of these treatments and the distinguishing and overlapping clinical features of these disorders is presented in
Table 1.
A general principle of management is prevention of sleep-related injury to the patient or bed partner. Inquiry into current or previous injuries facilitates risk assessment and prompt implementation of safety measures, such as removing dangerous items near the bed (furniture with sharp corners or weapons), lowering the mattress closer to the floor, installing bed rails, or placing pillows or other padding on the floor around the bed (
27,
28,
30). Timely recognition and treatment of RBD mimickers, such as obstructive sleep apnea or periodic limb movement disorder, may theoretically influence the severity and frequency of dream enactment behaviors (
27).
Current PTSD management strategies emphasize manualized trauma-focused psychotherapies (e.g., prolonged exposure or cognitive processing therapies) over pharmacological or other psychological therapies. Pharmacotherapies or non-trauma-focused psychotherapies are recommended as second-line treatments if trauma-focused therapies are unavailable or not preferred. Currently, SSRIs (sertraline, paroxetine, fluoxetine) or venlafaxine are considered first-line pharmacological treatments (
2). However, for patients with PTSD and antidepressant-induced dream enactment behaviors or RBD, psychotherapies may be preferred treatment options, especially considering their relative efficacy for PTSD (
14). Although bupropion may be less likely to induce RSWA and RBD, its role in treating PTSD without comorbid depression is limited (
2).
Guidelines for management of PTSD-related insomnia and nightmares also favor psychotherapeutic over pharmacological interventions. Cognitive-behavioral therapy for insomnia may treat both insomnia and nightmares, while imagery rehearsal therapies target the latter (
2,
32). Despite conflicting data regarding its efficacy, prazosin is commonly used for nightmares associated with PTSD (
33). Mysliwiec et al. (
11) proposed prazosin as an effective treatment for TASD based on their clinical experiences. However, diagnostic criteria and related treatments for TASD require further validation.
In contrast to PTSD, management of RBD typically involves pharmacological interventions, especially if bedtime safety measures cannot ameliorate injury risk. Clonazepam, dosed at 0.25–3 mg and administered 30–60 minutes before bedtime, is widely used. However, clonazepam may worsen obstructive sleep apnea symptoms and, in elderly patients, fall risk and cognitive impairments (
27,
30). Melatonin, at doses ranging from 3 to 12 mg at bedtime, is preferred over clonazepam due to its relative safety and tolerability. Evidence supporting other pharmacological interventions for RBD, including acetylcholinesterase inhibitors, melatonin receptor agonists, and dopamine receptor agonists, stems from case reports or series (
27,
30). Of note, the medications listed here for RBD are not preferred treatments for PTSD (
2).
Current Management Pitfalls
Uncertain relationships between dream enactment behaviors, PTSD, RBD, and neurodegeneration and their associations with other sleep disorders set the stage for disjointed clinical assessment and management. Our example of a patient with dream enactment behaviors, PTSD, and Parkinson’s disease highlights the importance of approaching PTSD-associated dream enactment behaviors from a transdisciplinary perspective that includes the broad differential diagnosis.
Treatments initiated in isolation for either PTSD, RBD, or other sleep disorders and without necessary diagnostic information from a neuropsychiatrically informed comprehensive sleep history and video polysomnography are likely to be biased by the lens of a particular clinician’s professional specialty (e.g., pulmonology, sleep medicine, neurology, psychiatry) and the focus it places on particular elements of the differential diagnosis and specific treatment approaches.
A comprehensive sleep history that includes informant data aids in the diagnosis of RBD and may clarify the differential diagnosis, as discussed above (
34). That said, even when bed-partner data are available, the clinical reality is that differentiating nightmares from night terrors and other disruptive behavioral events based on history alone can be challenging and inaccurate. This is related in part to assumptions by patients, bed partners, and clinicians as to the etiology of the movements and vocalizations.
Cognitive impairments due to medications, neurodegenerative disorders (e.g., Parkinson’s disease), or TBI may further confound the ability to obtain an accurate history of sleep-related behaviors. For instance, when prescribed for a presumptive diagnosis of RBD, clonazepam could impair the patient’s ability to recall dreams and other history accurately and reliably. Furthermore, regardless of age and pre-existing cognitive impairments, benzodiazepine medications are not first-line therapies for PTSD (
2). Early consultation with a sleep specialist, diagnostic polysomnography, and collaboration among specialties may prevent potential adverse consequences of prescribing benzodiazepines empirically for a presumed diagnosis of RBD and comorbid PTSD symptoms.
Antidepressant-induced exacerbation of dream-enactment behaviors and confounds on polysomnographic assessment of muscle tone during REM sleep further complicate assessment of PTSD-associated nocturnal behavior disturbances. The International Classification of Sleep Disorders–Third Edition (ICSD-3) requires that diagnosis of RBD not be accounted for by a medication effect, except that medications may unmask latent RBD in individuals with preexisting RSWA (
35). However, antidepressants used to treat mood disorders and PTSD are reported to induce RSWA, as previously discussed (
14,
29). Further, higher rates of depression and anxiety disturbances before and over the course of Parkinson’s disease, while likely to increase the prospect that antidepressants will be prescribed, are also thought to be premotor manifestations of the underlying neurodegenerative process (
36). Accordingly, this sequence of events may support a link between PTSD, RBD, and Parkinson’s disease.
The polysomnographic diagnosis of RBD, however, may be hindered by antidepressant medications, which suppress REM sleep (
37). Hence, before initiating treatment with a benzodiazepine or an antidepressant, a sleep-focused history with referral for attended video polysomnography is an important step to more clearly establish the diagnosis of RBD and more fully inform clinical management of this or similar conditions.
Future Directions
As stated previously, longitudinal studies are required to clarify potential relationships between PTSD, RBD, and neurodegeneration risk. The investigations of representative cohorts may incorporate systematic assessment of dream enactment behaviors, presence (or absence) of PTSD and other psychiatric disorders, sleep-related symptoms, medication and substance-use exposures, and autonomic dysfunction, parkinsonism, olfaction abnormalities, and other prodromal markers of synucleinopathies. Development of validated, portable polysomnography devices with mechanisms for sleep staging and EMG monitoring would greatly enhance the feasibility of RBD diagnosis and outcome monitoring in these cohorts (
14).
It is possible that REM atonia in PTSD, and possibly TASD, is related to a shared neuropathophysiology with RBD. Proposed mechanisms include amygdala hyperactivity or injury to the locus coeruleus-subcoeruleus complex altering atonia during REM sleep (
13,
14). Neuroimaging studies examining structural integrity functional connectivity of these regions in patients with PTSD (or TASD) and dream enactment behaviors may elucidate shared neural pathways contributing to RSWA.
Finally, the effect of treatments for PTSD symptoms on RBD severity or vice versa is not definitively established, except that antidepressants may elicit RBD in susceptible individuals. Identifying markers of susceptibility to antidepressant-induced RBD may enhance treatment algorithms and outcomes for patients with PTSD. Finally, the proposed criteria and treatments for TASD also require further validation (
12).
Conclusions
In both clinical practice and research, diagnosis and management of dream enactment behaviors in patients with PTSD require a transdisciplinary and collaborative approach. The broad differential diagnosis of dream enactment behaviors in PTSD underscores the importance of comprehensive assessment and consideration of the risks of medication interventions when the emotionally traumatic event is assumed to have an etiological role in the sleep disturbance. The common use of antidepressants for PTSD and related psychiatric disturbances also complicates diagnostic evaluation and treatment selection; the timing and interpretation of video polysomnography evaluation for RBD need to consider this complicating factor. Informed by the issues highlighted in this article, research on the pathophysiologic and prognostic relationships between PTSD, RBD, trauma exposure, and other conditions associated with neurodegeneration will improve diagnostic accuracy and treatment outcomes in the subpopulations of patients with combinations of these comorbidities.