There is a growing body of research on auditory processing disorders in mental and neurological diseases, such as schizophrenia (
1–
4), autistic disorders (
5), alcohol addiction syndrome (
6,
7), attention-deficit hyperactivity disorder (
8), anorexia (
9), dyslexia (
10–
12), and Alzheimer’s disease (
13–
15). The relationship between depression and hearing has also been identified (
16–
20). In most of these studies, participants listened to single tones, and the research addressed the process of hearing; only a few studies focused on the process of speech understanding.
The process of speech understanding occurs in several places that differ anatomically and physiologically; it also involves processes not directly related to the auditory system, including visual, motor, and other nonlinguistic cognitive functions (
21). Correct speech understanding depends on correct sensory perception, for which the physical performance of the ear is essential, and on the processing of auditory information occurring on the upper levels of the auditory pathway (
22).
The process of speech understanding under adverse acoustic conditions is increasingly being studied. This approach allows traditional speech processing models to be complemented by a range of processes connected with everyday speech (
23). In the surrounding environment, communication is often difficult because signals that would be audible under normal conditions can be masked by coexisting interference. In extreme cases (with very low signal-to-noise ratios), as a result of masking, the speech signal cannot effectively stimulate the nerve receptors that it would normally stimulate, as these receptors are already responding to much more intense noise. Usually, two types of noise used in research are distinguished (i.e., information noise and energy noise) (
24,
25), although recent works also identify modeling noise (
26). Information noise contains linguistic information resembling target speech, whereas energy noise is interference from nonspeech noise sources. Depending on the type of noise used in the research, the results vary, as different types of noise affect other parts of the auditory system (
27). Studies on speech understanding in noise conducted so far show that people with more severe symptoms of depression have more difficulty with informational interference (
28,
29).
The primary aim of the present study was to assess the understanding of speech in noise in people with unipolar depression and bipolar disorder during a mood episode (mania or depression) and during remission, compared with healthy people. The second objective was to investigate the relationship of these disorders with the stage of the disorder and demographic and clinical factors, including hearing impairment. We used energy masking, which is less prone to executive function deficits (
30).
METHODS
This study was approved by the Bioethics Committee of Poznań University of Medical Sciences. All of the participants provided informed consent after the nature of the procedures had been fully explained.
Study Participants
The study included 110 persons. Seventy-five (male, N=25; female, N=50; mean age=42 years [SD=14]) were patients with diagnosed mood disorders, hospitalized during the study at the Adult Department of Psychiatry, Poznań University of Medical Sciences. Forty-three patients had bipolar disorder (mania, N=20; depression, N=23), and 32 had unipolar depression. The diagnosis was made for each patient according to the International Classification of Diseases–10th Revision and DSM-5 criteria. The severity of symptoms was evaluated with the 17-item Hamilton Depression Rating Scale (HAM-D) (
31) and the Young Mania Rating Scale (YMRS) (
32).
Thirty-five persons (male, N=20; female, N=15; mean age=39 years [SD=14]) comprised the control group. Healthy volunteers were qualified for the control group. The exclusion criterion for all groups was the existence of severe and unstable somatic disease. Additionally, the exclusion criterion for the control group was the occurrence of any psychiatric disorders (including unipolar depression or bipolar disorder), presently and in the past.
Evaluation of Speech Understanding
The measurement tool in the study was the Polish Sentence Matrix Test (PTZ-M). PTZ-M is used to measure speech intelligibility in noise. It is a matrix test and is based on a limited lexical basis. During the test, the lexical material is presented against the background noise. Speech is masked using an energy marker. PTZ-M (
33) determines the SRT. SRT is defined as the minimum intensity needed to understand 50% of the spoken words expressed in decibels. The accuracy of SRT measurement is crucial because only the result of a reliable “speech in noise” test gives an insight into the subtle differences between SRT obtained under different conditions (before and after therapy) (
22).
The interpretation of SRT results from the physical definition of the following index: SRT is equal to 20 lg (from the ratio of the speech signal strength to the masking noise strength). If in the speech intelligibility test the values of speech intensity and masking noise are equal, then the ratio of these intensities is 1. The logarithm of 1 is 0 dB, which multiplied by 20 is 0 dB. An SRT ratio of 0 dB means that 50% of speech intelligibility occurs when the intensities of the speech and noise signal are equal, and correct speech intelligibility can be inferred. On the other hand, if the intensity of the speech signal must be increased by the listener above the voltage of the interfering noise (e.g., it is twice as high as the intensity of the masking noise, which occurs when a high level of external interference is observed during the measurement due to significant hearing loss of the patient or as a result of cognitive impairment), then the result is as follows: SRT=20 lg[2/1]=20(0.3)=6 dB (positive decibels). This means that if the intensity of the speech signal must be greater than the noise, the SRT will be in the positive decibel range (the greater the positive decibels, the worse the intelligibility and the more external factors affect intelligibility). If the intensity of the speech signal is less than the intensity of the noise signal (to achieve 50% intelligibility, such as can be achieved with good hearing, no pathology, in a quiet environment, etc.), then the SRT will be expressed in negative decibels. In other words, the more negative decibels expressing the SRT, the less impact of external noise, hearing pathology, mental disorders, and so forth on speech intelligibility.
Audiological Evaluation
For the audiological diagnosis, the Madsen OtoFlex 100 (Otometrics, Copenhagen) clinical impedance audiometer and the Madsen Aster (Otometrics) clinical tonal audiometer were used, with an extended upper-frequency range, equipped with Sennheiser HDA200 headphones. Impedance audiometry included tympanometry and measurement of the intra-aural muscle reflex, assessing the middle ear function. This made it possible to diagnose the conductive components of hearing loss. Pure tone audiometry was performed in the conventional standard 125 Hz–8,000 Hz frequency range and the high-frequency band (high-frequency pure tone audiometry) to 16 kHz, with half-octave accuracy.
Test Procedure
By using a cross-sectional design, study 1 examined the understanding of speech expressed by the SRT index in persons with a mania episode in the course of bipolar disorder (N=23), with a depression episode in the course of bipolar disorder (N=20), and with a depression episode in the course of unipolar depression (N=32). In study 2, the same variable was measured after a mood episode subsided during remission. Patients in the control group (N=35) were examined once.
For patients in the bipolar disorder with mania group, the episode criterion was severity of symptoms ≥20 points, and remission ≤7 points, on the YMRS; for patients with depression, both bipolar and unipolar, the episode criterion was severity of symptoms ≥18 points, and remission ≤7 points, on the HAM-D.
Additionally, clinical patients in the remission phase underwent audiometric tests in the audiological laboratory of the pediatric otolaryngology clinic to diagnose hearing disorders. Hearing impairment was diagnosed in seven patients with unipolar depression.
Statistical Approach
The Shapiro–Wilk test was used to check the compatibility of the distribution with the normal distribution. For correlation analysis, Pearson’s correlation (bipolar disorder with mania group and unipolar depression group; distribution consistent with normal distribution) and Spearman’s rank correlation (bipolar disorder with depression group; distribution inconsistent with normal distribution), respectively, were used. The Wilcoxon test (distribution inconsistent with normal distribution) was used to investigate differences between two interdependent groups, the Mann–Whitney test (the distribution deviates in shape from a normal distribution) was used to investigate differences between two independent groups, and analysis of variance (Kruskal–Wallis test) was used to investigate differences between a larger number of independent groups. For this test, post hoc tests were also performed. To analyze the relationships between many independent variables and a dependent variable, a multiple regression analysis was carried out.
Because of the large scattering of the results obtained and the fact that the SRT is expressed in decibels (i.e., logarithmic rather than arithmetic value), values such as medians and range (minimum and maximum value) were taken into account in the analysis of the results.
The statistical analysis was performed using the STATISTICA-10 program. The significance level was set at a p value <0.05.
RESULTS
Demographic and clinical characteristics of the study groups are presented in
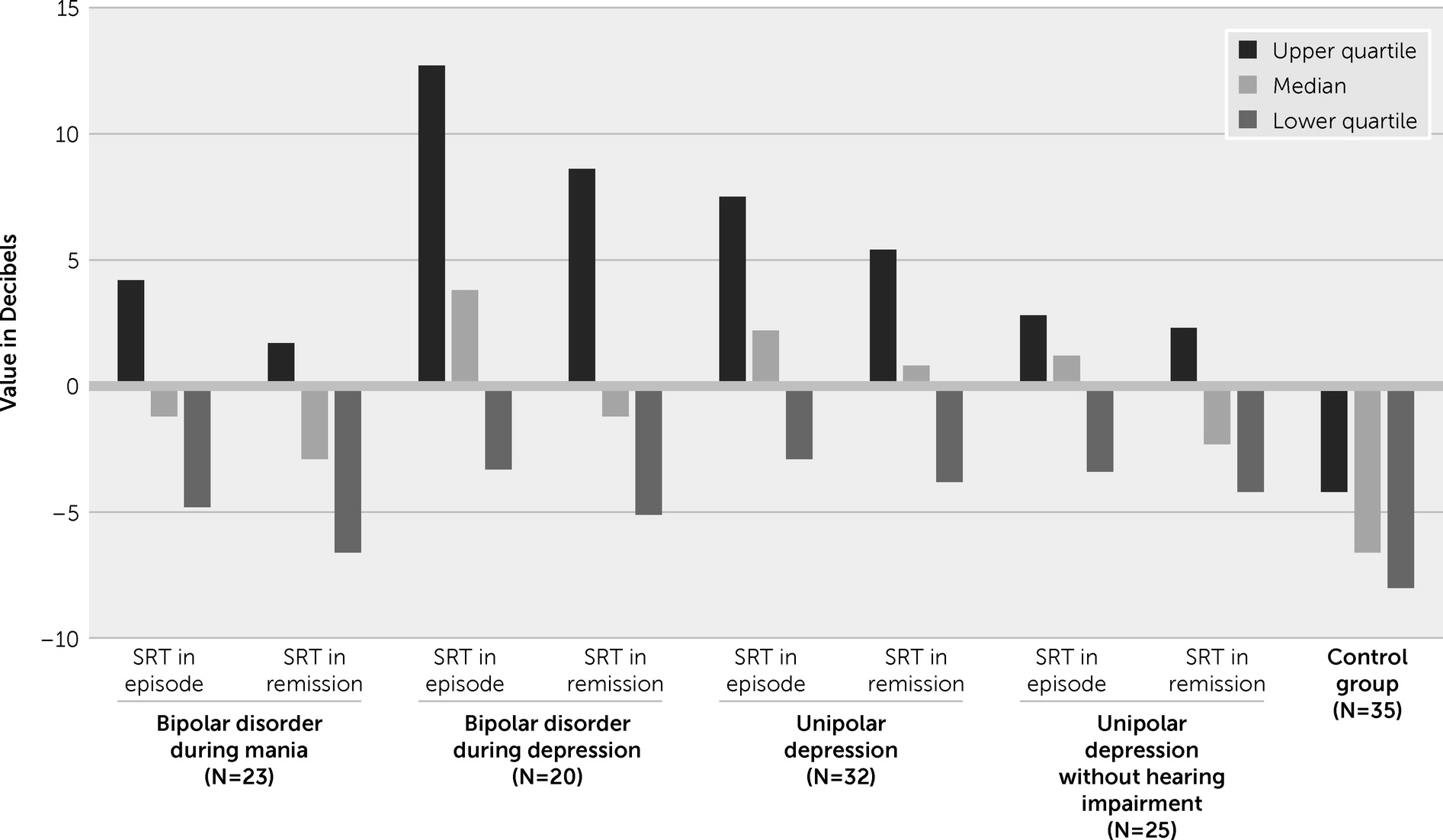
Table 1. The study subjects did not differ significantly in terms of demographic and clinical characteristics. The results of the SRT index obtained are presented in
Figure 1. Detailed comparison of the individual groups is presented in
Tables 2–5. The SRT differences in the clinical and control groups are summarized in
Table 2. The differences in the SRT obtained during the analyses were visible both in the first study, during the mood episode, and in the second study during remission.
Comparison of individual groups using post hoc tests showed that there were significant differences between the clinical and control groups. Patients with bipolar disorder during a mania episode achieved higher SRT values than patients in the control group (p<0.01). Patients with bipolar disorder during mania remission achieved higher SRT values than patients in the control group (p<0.05). Patients with bipolar disorder during a depression episode achieved higher SRT values than patients in the control group (p<0.05). Patients with bipolar disorder during depression remission achieved higher SRT values than patients in the control group (p<0.01). Patients with unipolar depression during an episode achieved higher SRT values than patients in the control group (p<0.01). Patients with unipolar depression during remission achieved higher SRT values than patients in the control group (p<0.01). SRT values of patients during a mood episode and in remission are presented in
Table 3.
Bipolar disorder patients during a mania episode and after reaching remission presented a similar SRT index (they understood speech at a similar level). In contrast, during a depression episode, bipolar disorder and unipolar depression patients had a higher SRT compared with that measured during remission (they understood speech less well during the episode than after achieving remission). In patients without hearing loss, the difference in SRT levels between a mood episode and remission was close to the limit of significance.
Patients with depression, whether in the course of bipolar disorder or unipolar depression, achieved a similar SRT (they understood speech at a similar level), both when they were undergoing an episode (Mann–Whitney test p=0.58; Z=−0.55) and during remission (Mann–Whitney test p=0.94; Z=0.07). A similar SRT index was obtained in patients without hearing loss in both disorders during an episode (Mann–Whitney test p=0.14; Z=1.47) and during remission (Mann–Whitney test p=0.35; Z=0.73). The relationship between the SRT and demographic and clinical factors is shown in
Table 4.
The SRT in patients with a depressive episode in the course of bipolar disorder showed a correlation with the age of the subjects in the study, the duration of the disorder, the number of episodes and the number of hospital stays, and in remission with age and duration of the disorder. The SRT in the group of persons with bipolar disorder was associated with hearing loss during depression and remission. Hearing loss was diagnosed in seven patients in the group of patients with unipolar depression. This effect was taken into account in the analyses.
Table 5 presents the result of the regression analysis of the impact of demographic and clinical factors on the SRT index.
Multiple regression analysis showed that an increase of the age of bipolar disorder patients in a depressive episode by 1 year resulted in an average increase in the SRT by 0.621 dB (SD=0.193), with other variables remaining constant (56.8% of SRT variability). In bipolar disorder patients in depression remission, an increase in age by 1 year resulted in an increase in the SRT value by 0.434 dB (SD=0.166) on average, with the remaining variable remaining constant (43.3% of SRT variability). The other factors proved to be irrelevant for the SRT.
DISCUSSION
The results of our study are consistent with those of others, which indicate poorer understanding of speech against a backdrop of noise in depressed persons compared with healthy persons (
28,
29). In addition, this study demonstrates that this effect persists even after a mood episode has subsided. This has practical implications because speech perception in typical social conditions is often disturbed by noise, leading to communication problems, and may exacerbate social difficulties for patients with mood disorders.
Differences Between Mania Episode and Mania Remission
An important result of our study is showing that an episode of mania does not affect deterioration of speech understanding compared with mania remission. Mania, as opposed to depression, may not significantly impair the processing of stimuli relevant to speech understanding. This may be due to several factors. First, previous fMRI studies have shown that the loudness dependence of the auditory evoked potentials (LDAEP; N1/P2 component) generated by the primary and secondary auditory cortex are altered in patients with depression. In response to prophylactic treatment with lithium and serotonin reuptake inhibitors, elevated LDAEP returns to normal. It has been suggested that serotonergic neurotransmission, which plays a role in the neurobiology of mood disorders, may be relevant for the auditory system (
16,
34). Thus, it is possible that speech understanding, which is impaired in depression but not in mania, is associated with an increase or decrease in serotonin levels, the amount of which remains altered depending on the state of the disorder.
Secondly, a study on the integration of visual and acoustic stimuli involved in speech perception in persons with bipolar disorder has shown that persons in a manic episode do not perform worse in acoustic-visual and visual perception than the control group. On the other hand, patients with bipolar disorder in a depressive state showed worse visual integration. Researchers have suggested that this integration is related to behavioral and cortical information processing; the results indicate that the manic state in this study also did not disrupt the processes associated with the audiovisual integration of perceived speech (
35).
Meta-analyses on the cognitive functioning of individuals with bipolar disorder indicate that during a mania period there are numerous cognitive deficits and disorders that persist during remission, mainly connected with prefrontal cortex function (in terms of attention span and some aspects of the executive functions: response inhibition, planning, problem solving) (
36–
39). Nevertheless, reports indicate that the state of mania results in their more efficient functioning of certain cognitive functions. A study on social cognition has shown an increase in affective empathy (overempathizing) during a manic episode as compared with depressed persons and the control group. This phenomenon does not occur in the case of cognitive empathy. It may be related to disorders of emotion inhibition associated with anastrophic thinking and increased activity of mirror neurons during a manic episode (
40). Other studies have shown a higher level of creativity in persons with bipolar disorder compared with healthy subjects; higher levels were also shown in children with bipolar disorder having a family history of this disorder (
41). The state of hypomania in particular favors increased creativity. It may result from increased fluidity, speed, and excessively inclusive thinking occurring during a period of elevated mood, as well as increased activity and tendency to act in such states (
42). Our study did not show that patients in mania understand speech better than healthy persons; further research is required involving comparison with the control group. This would also allow for the clarification of a specific cognitive profile that is characteristic of mania.
Differences Between a Depression Episode and Depression Remission
Our research confirms that an episode of depression negatively affects the understanding of speech presented against the background of noise. Previous single-tone studies have shown that a state of depression can impair the functioning of the auditory system (
16–
20). Studies concerning the understanding of speech rather than sounds have shown that in persons with depressive symptoms, there are differences between strong and weak depressive symptoms if speech is masked by informational noise, whereas these differences have not been observed when energy noise was used (
28). Energy noise remains more resistant to attention processes (
23) and does not require so much involvement of the executive functions (
30), which remained impaired in the event of depression (
43). In our study, energy noise was used, which may indicate that speech in depression remains disturbed regardless of other cognitive functions. This may also confirm the hypothesis that a poorer understanding of speech masked with information noise in depressed patients is associated with a higher susceptibility to information distraction in persons with unipolar depression (
29). However, such conclusions would require controlling other cognitive functions of subjects in further studies.
Effect of Demographic and Clinical Variables on Speech Understanding
Our study found no difference between the level of speech understanding in bipolar disorder and unipolar depression. The concept of the existence of specific cognitive profiles in mood disorders has been studied only in recent times. An assumption is that defining a precise profile could supplement or be an alternative to symptomatic diagnoses. The studies to date also have not explicitly identified a different cognitive profile between various mood disorders (
44). The results of our research indicate the absence of a different profile characteristic of a particular subgroup of mood disorders.
The effect of age, disorder duration, number of episodes, and number of hospital stays on speech understanding in bipolar disorder patients with depression is consistent with the results of studies that show that cognitive dysfunction in mood disorders worsens with age (
45), disorder duration, number of relapses, and number of hospital stays (
46). Additionally, it has been shown that speech perception in noise is influenced by education level, cognitive performance, and depressive symptoms. The better the cognitive level and the higher the education level and the lower the severity of depression, the better the recognition of speech in noise (
47).
Limitations
The general cognitive functioning of the subjects has not been tested, which makes it impossible to search for relationships between speech understanding results and other cognitive functions. A small number of subjects makes it difficult to generalize into a larger population. All subjects were treated pharmacologically, which may have influenced the results (
48). In order to better identify the relationship between speech understanding disorders and clinical change, it would be advisable to include persons in long-term remission and possibly assess speech understanding after a change from a mania episode to a depression episode in bipolar disorder patients. The examination of relatives of persons with mood disorders would allow us to answer the question of whether speech understanding disorders may be endophenotypes of these conditions. Another limitation of the study was connected with the considerable scattering of results. A large proportion of the results, which are far removed from the average, made it impossible to take this value as representative of the group. In order to reduce the impact of this limitation, median and quartile values were presented in the analysis of the results.
CONCLUSIONS
Patients with mood disorders understood speech worse than control subjects, regardless of whether they were in a mood episode or in remission. Manic episodes in the course of bipolar disorder were not associated with impaired speech understanding compared with mania remission. However, a depressive episode in the course of bipolar disorder was associated with poorer speech understanding compared with depression remission. The SRT in bipolar disorder with depression showed a correlation with age, disease duration, number of episodes, and number of hospital stays, and in remission with age and disease duration. A depressive episode in unipolar depression was associated with poorer speech understanding compared with unipolar depression remission, and this phenomenon was more severe in persons with impaired hearing.
Acknowledgments
The authors thank Edward Ozimek for the availability of the Polish Sentence Matrix Test.