Role of PV interneurons in Neuropsychiatric Disorders
A recent review by Ruden et al. (
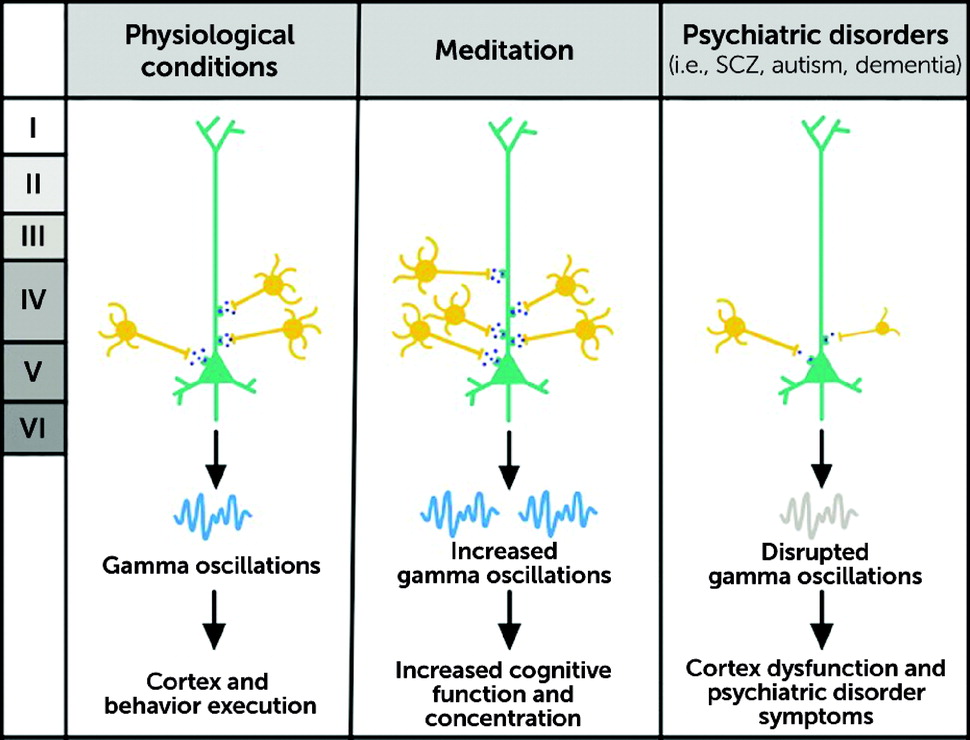
1) highlights the role of PV neurons in many neuropsychiatric disorders, including schizophrenia, Alzheimer’s disease, autism spectrum disorder (ASD), bipolar disorder, and epilepsy. The functions of the fast-spiking PV interneurons are heavily diminished in the PFC in numerous psychiatric disorders, including schizophrenia and ASD (
64). PV interneurons are powerful regulators of E-I balance in the PFC, and they help optimize the representation and processing of supramodal (i.e., independent of the sensory modality) information in the PFC. The voltage-dependent potassium channels (KV 3.1 and KV 3.2 types) which are located in the PV interneurons are reduced in number in untreated schizophrenia (
66) and presumably in ASD as well (
67). In dementia with Lewy bodies (DLB), an alpha-synucleinopathy, there is partial loss of the number of the PV interneurons due to long-term calcium overload and impaired mitochondrial functions, thus leading to deficits in the gamma-frequency activity (
68). The cognitive deficits in DLB due to dysfunction of dorsolateral PFC (DLPFC) networks are linked to disturbances in gamma-frequency oscillations (generated in the layer III of the DLPFC). Although the density of the cortical PV interneurons has not been observed to be altered in schizophrenia, the levels of expression of several proteins involved in the regulation of the functions of the PV interneurons, as detected in their transcriptome expression patterns, have been found to be lower in this severe mental illness (
69).
Because the PV interneurons are powerful regulators of the E-I activity in the PFC, reductions of their number or functioning can cause deficits in the E-I balance that alter the gamma-frequency oscillations. Such deficits are associated with anhedonia, social withdrawal, deficits in working memory, and diminished cognitive flexibility and control, which are seen in many psychiatric disorders, including schizophrenia, ASD, and major depressive disorder (
17,
70,
71). In schizophrenia, the PV interneuron inhibition onto the pyramidal cells is decreased (impaired gamma oscillations) which has been hypothesized to be a key locus of the pathophysiology underlying schizophrenia (
72,
73). The PV interneurons show marked decreases in size in both the cell body and the dendritic system in individuals with schizophrenia (
74). In a postmortem study that compared the hippocampal specimens of bipolar affective disorder patients with those from healthy control subjects, showed reduced volume of the nonpyramidal cell layers, reduced cell body volume, and reduced messenger RNA (mRNA) levels in PV interneurons in the bipolar disorder group (
75). Depression is associated with somatostatin-positive GABA-ergic interneurons in the DLPFC, whereas decreased PV interneuron mRNA levels appears to distinguish bipolar disorder from major depressive disorder (
76). From a therapeutic (disorder modifying) perspective, the GABA neurons, when exposed to serotonin (5-HT
1A) agonists and the neurotrophic agent T-817MA, have been proposed to prevent the onset or progression (or both) of schizophrenia (
77). As mentioned above, PV interneurons maintain a high level of inhibition through the mediodorsal thalamic inputs relative to excitation in pyramidal neurons (
64). By regulating spike timing and oscillatory patterns, they contribute to proper execution of PFC networks and, in turn, behaviors. It is speculated that either cell-autonomous factors or altered inputs can lead to multiple gene (presumably) pathologic expression patterns in PV interneurons that contribute to cognitive deficits in several psychiatric disorders, including schizophrenia (
78).
A study conducted in 12 patients with schizophrenia, compared with 12 healthy control subjects, found that neural circuit impairments resulted from failure of gamma-band synchronization (
79). In the first report of distinctive patterns of gamma activity in three symptom syndromes seen in patients with schizophrenia (defined as psychomotor poverty, disorganization, and reality distortion), 35 patients with schizophrenia were compared with 35 age- and gender-matched healthy control subjects, and the sample with schizophrenia showed generally reduced gamma activity. Analysis of the responses to 40 target and 40 nontarget auditory stimuli showed that reality distortion was correlated with increased gamma, whereas psychomotor poverty and disorganization were correlated with decreased gamma synchronicity, and the authors suggested that this may explain disturbances in integrated brain function characteristic of schizophrenia and provide complementary insights to the established slower frequency event-related potential dysfunctions in schizophrenia (
80). A 2012 review by Gandal et al. (
81) hypothesized that GABA-ergic circuit deficits—particularly the disruption of fast-spiking PV-expressing interneurons—result in pathophysiological deficits that are linked to core symptoms of schizophrenia. Furthermore, dysfunctional fast-spiking interneuron activity appears to be a final common pathway in schizophrenia that could explain gamma abnormalities in cortical inhibitory pathways. The review also speculates that gamma abnormalities are a biomarker or endophenotype for treatment-resistant symptoms of schizophrenia—most notably, negative symptoms—and might be used ultimately as a rational target for therapeutic intervention (
81). High prevalence of reductions in PV interneurons or reductions in GAD67 (GABA-ergic enzyme protein) expression in the mPFC are observed with decreases in task-evoked gamma oscillations, which is a recognized feature in schizophrenia. In schizophrenia, environmental factors, too, such as social isolation, are posited by some as pathoetiological factors that converge with genetic causative factors (multifactorial etiology). This could be linked to PV interneuron susceptibility to oxidative stress from these same environmental stressors (e.g., social isolation) because of downregulation of peroxisome proliferator-activated receptor-γ coactivator-1α, a transcription coactivator that is essential in cellular energy metabolism and has antioxidant properties (
82,
83).
Dysregulation of the GABA-ergic signaling has been implicated in the etiology of ASD, anxiety disorders, and epilepsy (
17). For example, a postmortem analysis of neocortical tissue from 11 patients with ASD and 10 matched control subjects that examined PFC PV interneurons detected significant reductions in the autistic patients compared with control subjects. This dysregulation of GABA-ergic signaling is posited to affect the balance of E-I and alter gamma-wave oscillations in the cerebral cortex of autistic subjects (
71). In some brain regions, animal studies have shown that the inhibition of PV interneurons reduces anxiety and improves cognitive performance. For example, in a mouse model, rhythmic brain stimulation of the ACC and inhibition of PV interneurons can lead to a reduction in anxiety and the formation of new myelin-producing oligodendrocytes (
84). Also, in a murine model, stimulation of PV interneurons in the dentate gyrus produced an anxiolytic effect, with an increase in learning and memory, and enhanced the extinction of fear-causing memories (
85).
Current research posits that the PV interneurons are implicated in many psychiatric disorders (
86). Stress can inactivate PV interneurons and accelerates the loss of their spiny dendritic processes. This might disrupt global neuronal excitation or inhibition in the primary somatosensory cortex and result in perceptual disturbance. Interestingly, pharmacogenetic and behavioral manipulations that enhance PV interneuron activity can prevent stress-induced dendritic spine loss and overexcitation (
87). Deep brain stimulation of thalamocortical neurons, for example, increased the activity of PV interneurons in brain cortex (
88). Disturbances in NMDA receptor (NMDAR) signaling in fast-spiking PV interneurons can induce cognitive deficits as well as psychosis-like symptoms in humans (
29). The newly investigated psychotropic with antidepressant effects, ketamine, produces at least some of its antidepressant action by means of inhibiting NMDARs expressed on GABA-ergic interneurons, leading to pyramidal cell disinhibition and an enhancement of excitatory glutamatergic neurotransmission in the medial PFC (mPFC) and, potentially, other mood-relevant corticolimbic brain pathways (
89,
90). Ketamine, notably at antidepressant doses, prevented stress-induced dendritic spine loss, increased PV interneuron activity, prevented net loss of PV axonal boutons, and modulated dendritic spine plasticity in response to stress (
91).
The cortical silent period (CSP), a measure related to transcranial magnetic stimulation (TMS) in meditators, found that there is a significant increase in the CSP linked to GABA-ergic cortical inhibition, a mechanism implicated in improved cognitive performance and enhanced emotional regulation (
92). Meditation-related increases in GABA-B seems to modulate cortical inhibition. A study of 40 undergraduate meditators demonstrated that 20-minute daily meditation sessions reduced fatigue, increased vigor, and produced a marked increase in stress-related cortisol and an increase in immunoreactivity. These meditation sessions also reduced anxiety, improved attention and cognition, and increased rhythmic electrical activity in brain areas related to emotional control (
93). Optogenetic technology in a mouse model found that inhibiting PV interneurons suppressed gamma oscillations and, conversely, that stimulating PV interneurons generated gamma-frequency rhythmicity (
94). However, despite promising lines of inquiry using even powerful new genetic engineering techniques, it remains unknown if these deficits in gamma synchronicity in humans can be clinically and therapeutically corrected so as to demonstrate meaningful clinical improvements. These pieces of evidence all converge to suggest that PV interneurons are involved in basic but essential functions that help regulate somatic and mental states. In essence, and a promising finding, meditation and mindfulness techniques produce neurobiological changes in the brain and physiologic improvements in body function that have been shown to be enduring for patients who continue to practice these techniques (
39). More human studies are necessary to ascertain the exact roles of PV interneurons and gamma oscillations in these states and various psychiatric disorders.