Although the insula was described by Brodmann and von Economo early in the 20th century, there has been a recent surge in interest in this area. Comprising only approximately 2% of cortical surface area, research over the last two decades supports participation of the insular cortex in a wide range of functional circuits.
9 There is growing evidence that the insula is important to neuropsychiatric disorders, especially in relation to dysfunctions involving higher order cognitive, emotional, and social networks. The insula’s primary role is believed to be that of multimodal integration. With this increased research attention, knowledge regarding the insula’s role in clinical syndromes has also increased.
Microscopic Structure
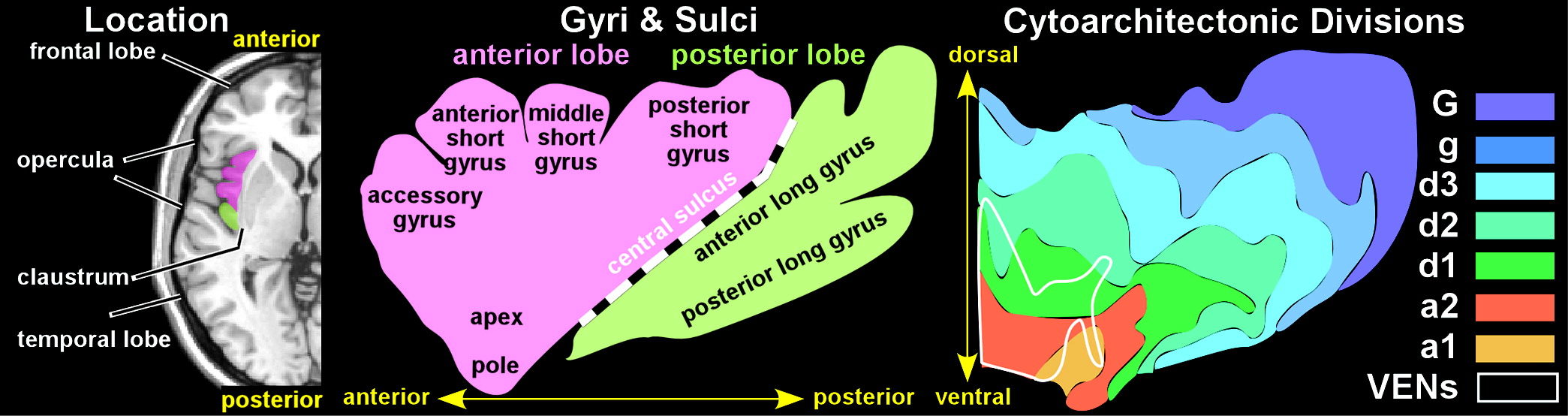
The insula is isocortex (neocortex) comprised of six distinct layers. Brodmann divided the insula into two regions, separated by the central sulcus: an anterior agranular region (containing pyramidal neurons in layers II and IV) and a posterior granular region (containing granular cells in layers II and IV).
2 Later studies led to the development of a concentric model in which the agranular region is located ventral-anteriorly and the granular region is located dorsal-posteriorly, separated by a large band of dysgranular cortex.
2 Although there is both species and interindividual variability, the concentric model has been generally supported by more contemporary research.
1,12,13 Structural parcellation of the primate insula is an area of active research and debate. Recent studies identified seven distinct subdivisions in the human insula (
Figure 1) and 15 in the macaque monkey.
1,14An unusual feature of the insula is the presence of large bipolar (spindle shaped) projection neurons called von Economo neurons (VENs).
1,2,15 These are most common in the very anterior region of the insula (also called frontoinsular cortex) (
Figure 1). VENs are much larger than nearby pyramidal neurons and have symmetric long narrow apical and basal dendritic arbors.
16 On the basis of their size, researchers have suggested that VENs project to other areas of the brain as a fast relay transmission system. Postmortem studies in humans indicate that VENs primarily express transcription factors consistent with involvement in interoceptive functions (e.g., pain, immune, visceral) and subcortical projection targets (e.g., striatum, superior colliculus, basal pons, spinal cord).
17,18 Coexpression of other transcription factors was also found, which may indicate secondary projections to intracortical areas.
18Functional Anatomy
Multiple types of evidence and different types of data analyses have elucidated understanding of the heterogeneous and complex functional anatomy of the insula. Multiple impairments have been reported following injury to the insula in humans, including alterations in autonomic functioning, sensory (e.g., gustatory, olfactory, auditory, somatosensory, multimodal) functioning and body awareness, emotional functioning, and language.
19,20 Blood supply to the insula is from a main segment of the middle cerebral artery, so isolated insular stroke is unusual.
19 Improvement in empathetic functioning has been reported following resection of insular gliomas.
21The starting point for a more detailed understanding of functional anatomy in the human is provided by studies in nonhuman primates. It is important to bear in mind, however, that differences are apparent both when comparing across species of nonhumans primates and from nonhumans primates to humans.
22,23 For example, a study comparing of human tractography with monkey tract tracing found that, although a number of tracts were consistent across species, notable differences were observed in others (e.g., arcuate fasciculus, inferior fronto-occipital fasciculus).
24 Moreover, intraoperative electrophysiological studies suggest vastly increased reciprocal intrainsular connectivity in humans compared with nonhumans primates.
22Structural Connectivity
In nonhuman primates, evidence supports a posterior to anterior organization of sensory-related areas within the dorsal insula and a limbic-related area in ventral insula.
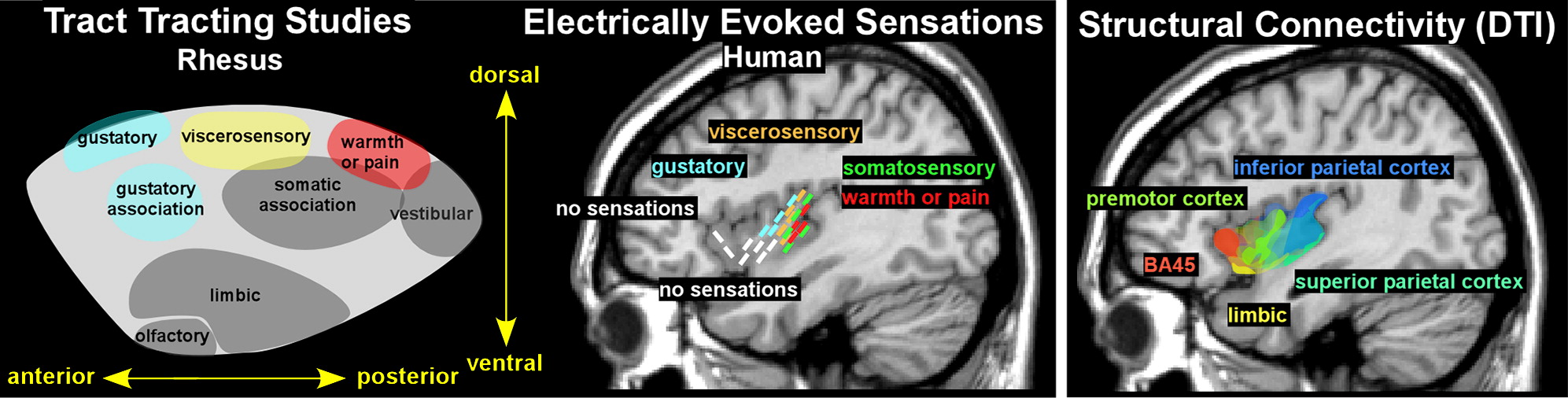
2,25 In the rhesus monkey, projections from vestibular and somatosensory areas go to posterior insula, viscerosensory input goes to midinsula, and gustatory input goes to anterior insula (
Figure 2).
2 Input from limbic-related areas (e.g., amygdala, temporal polar, parahippocampal, orbitofrontal, and anterior cingulate cortices) goes to the ventral insula (
Figure 2).
2 In the awake macaque monkey, stimulation of the dorsal posterior insula evoked sensorimotor responses (simple movements of the mouth, face, hand, or limbs).
25 Stimulation of the dorsal anterior insula evoked eating-related responses (chewing, mouthing), whereas stimulation ventral to the eating-related region evoked disgust responses (upper lip curling, nose wrinkling, food refusal). Stimulation in the middle region of the ventral insula evoked affiliative behaviors (lip smacking), but only when there was simultaneous eye contact between the monkey and experimenter.
25Two recent studies using intraoperative electrophysiology in patients with intractable epilepsy provide evidence for a similar posterior to anterior functional organization, but with sensory areas concentrated mostly within posterior insula (
Figure 2).
3,26 In one study, depth electrodes were used to deliver stimulation to various regions within the insula sufficient to induce symptoms.
3 The most posterior locations evoked somatosensory symptoms, with sensations of warmth and/or pain localized more dorsally (
Figure 2). Central locations evoked viscerosensory symptoms, with gustatory sensations localized more anteriorly. Anterior locations did not evoke consistent symptoms.
3 In the other study, depth electrodes were used to deliver stimulation below threshold for sensations but sufficient to induce cortico-cortical evoked potentials (recorded at other depth electrodes).
26 Most of the expected connections between the posterior dorsal insula and sensorimotor areas (e.g., motor, somatosensory and parietal cortices) were confirmed, but not all (e.g., posterior cingulate and supplementary motor cortices). Expected connections between the anterior dorsal insula and cognitive-emotion related areas were also confirmed for some (e.g., hippocampus, temporal pole, frontal operculum, orbitofrontal cortex) but not others (e.g., amygdala, entorhinal and anterior cingulate cortices). As noted by the authors, differences from what has been reported for nonhuman primates could be true species differences and/or a result of low sampling.
26Several groups have used diffusion tensor imaging–based tractography to explore the structural connectivity of the insula in humans.
4,27–30 The study that used clustering (k mean) to divide the insula into two functionally distinct regions based on similarities in structural connectivity (strength of connections to other areas) noted that the division between the posterior and anterior subdivisions occurred in the region of the middle short gyrus, following neither expected cytoarchitectonic or gyral boundaries.
28 The study that used seven insular regions of interest (dorsal portions of the anterior short, middle short, posterior short, anterior long, posterior long gyri; ventral anterior and ventral posterior) as seeds for tractography identified three subdivisions that agreed more closely to the results of tract-tracing studies in nonhuman primates.
27 The anterior region (anterior short gyrus and anterior ventral insula) was primarily connected with orbitofrontal, inferior frontal, and anterior temporal cortices. The posterior region (posterior long gyrus and posterior ventral insula) was primarily connected with temporal cortices. Between was a transition area (middle short, posterior short, anterior long gyri) with mixed frontal and temporal connectivity.
27 A study that used voxel-based probabilistic tractography and a different clustering technique (Laplacian eigenmaps) found the expected differences in patterns of connectivity between the posterior and anterior insula but also reported a more complex progression that is reminiscent of cytoarchitectonic mapping (
Figure 2).
4 Transitions in connectivity were gradual rather than abrupt, and individuals differed considerably in extent and locations of subdivisions. The general anterior-posterior and dorsal-ventral patterns of connectivity were mostly in agreement with expected connections based on nonhuman primates. Although no human diffusion tensor imaging studies have yet confirmed connections to the anterior cingulate cortex known to be present in at least some nonhuman primates, this may be due to the present limitations of tractography.
4,30Functional Connectivity
Whereas structural connectivity studies provide evidence of anatomical connections between brain areas, functional connectivity studies examine which brain regions coactivate (based on time series data) with the area of interest (seed region or voxel) during either task-free (resting state, also called intrinsic functional connectivity) or task-activated conditions. There are multiple differences across studies (e.g., subject selection and number, equipment and data acquisition methods, and data analytic decisions) that make comparisons of results challenging. For these reasons, meta-analyses based on querying the large databases of neuroimaging studies (e.g., NeuroSynth, BrainMap, and 1000 Functional Connectomes Project) that are now available provide a more robust method of examining insular functional connectivity than reviewing single site studies alone.
Resting State Connectivity
Although both seed- and voxel-based approaches have been used to subdivide the insula based on similarities in resting state functional connectivity, a strength of voxel-based approaches is the potential to provide more detailed parcellations.
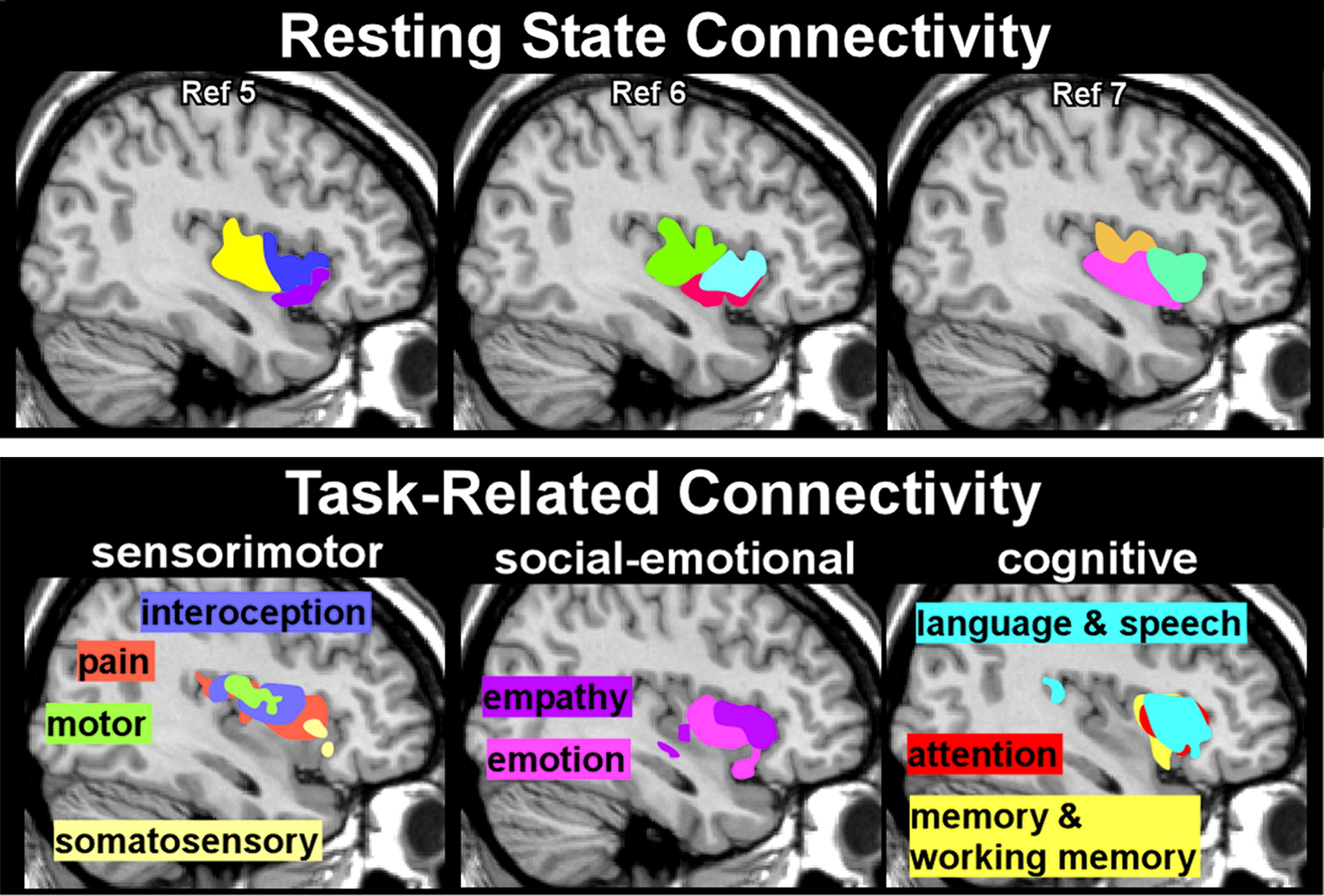
5–7,31 One meta-analysis agreed with an earlier single site study that three subdivisions (dorsal anterior, ventral anterior, posterior) were the optimal solution, although there were differences in the anatomic boundaries reported (
Figure 3) and in the areas reported to be functionally connected to each subdivision.
5,7 The other meta-analysis examined multiple solutions (2–15 subdivisions) and identified several that had high consistency.
6 The authors suggested that a number of valid models of functional differentiation can be obtained depending on level of specificity desired.
6Task-Related Connectivity
As noted above, this type of connectivity identifies areas that are simultaneously active during a specific task. It is important to bear in mind that an area within the insula might be activated only by a specific functional domain of tasks (low diversity, suggesting functional specificity) or might be activated by a wide range of task domains (high diversity, suggesting multimodal integration and/or domain-general functions).
32 Similarly, a specific functional domain of tasks may be associated with activation within one region or within several. Several studies have used the three subdivisions defined in resting state connectivity studies, clustering higher cognitive functions (e.g., executive control) with the dorsal anterior subdivision; emotional processing, chemosensory (e.g., gustatory, olfactory), and autonomic functions with the ventral anterior subdivision; and sensorimotor and language functions with the posterior subdivision.
5,7,32 However, the most recent of these meta-analyses noted that all 32 BrainMap task domains engaged each of the three putative subdivisions some of the time, suggesting that a more nuanced approach is required.
32Some studies have grouped tasks into functional domains and identified functional subdivisions by where the task-related activations occurred.
6,8,32 One meta-analysis focused on the 13 functional categories (emotion, empathy, olfactory, gustatory, interoception, pain, somatosensation, motion, attention, language, speech, working memory, memory) most frequently associated with activations within the insula. They grouped these into four domains (social-emotional, olfacto-gustatory, cognitive, sensorimotor) to identify four subdivisions with functional specificity.
8 No area was identified that activated to all functional domains. A small area in the dorsal anterior insula was identified as supporting multimodal integration because it was activated across all domains other than somatosensory and motor. Another meta-analysis assessed both resting state functional connectivity and task-related connectivity based on BrainMap’s six primary behavioral domains (action, cognition, perception, emotion, interoception, pharmacology).
6 Evaluation of cross-modal agreement between these two types of functional data indicated that agreement was best for two, nine, and 13 subdivisions. The authors emphasized that there was a high degree of overlap in subregions activated by particular behavioral domains, consistent with presence of integrative functions (
Figure 3).
6A number of theories have arisen that attempt to unify the functional entirety of the insula. In Craig’s “sentient self” model, information moves anteriorly across the insula.
33,34 A broad range of somatotopically organized interoceptive information is received by the posterior insula. Moving to the midinsula, the interoceptive information is integrated with input from higher sensory cortices and the limbic system. This stage of processing adds homeostatic information and emotional salience to the interoceptive representation. In the anterior insula, the representation is further enriched by integration of input from a large number of cortical areas, forming a unified coherent representation that may be the neural basis for the sense of self. Menon and Uddin
35 proposed that the insula functions in identifying salient external events, modulating the switch from basic attention to working memory and the activation of the sympathetic nervous system, and initiating the anterior cingulate cortex to quickly generate a motor response. Nieuwenhuys
2 proposed that the anterior insula together with the anterior cingulate comprise a “core control network that guides all mental activity and behavior in humans.” (p. 132). It has also been proposed that the insula subsumes two main meta-functions: an emotional-salience/attentional control network and a skeletal-motor integration network.
31 In any case, more research is needed, and sources seem to agree that the insula is involved in complex and multimodal functions.
Neuropsychiatric Disorders
In addition to having a wide range of functions, the insula has a prolonged developmental course that continues well into adolescence.
29 Thus, it is not surprising that this area has been implicated in many neuropsychiatric disorders. Studies in specific disorders are helpful in understanding the insula’s contributions to behavior.
Frontotemporal dementia (FTD; now called frontotemporal neurocognitive disorder) refers to several related conditions resulting from frontotemporal lobar degeneration. The anterior insula is particularly vulnerable to pathological processes seen in FTD, most notably with behavioral variant FTD (bvFTD), in which gray matter loss in frontal and anterior insular regions has been shown early in disease progression.
36 Poor judgment, impulsivity/disinhibition, and lack of empathy are clinical features of bvFTD, deficits consistent with the functions of the anterior insula region. Additionally, VENs, which are found primarily in anterior insular and anterior cingulate cortices, seem to be at increased vulnerability in this disorder.
37 Loss of these specialized neurons may help explain the clinical manifestation of bvFTD.
37 In a small but interesting study comparing patients with bvFTD to patients with Alzheimer’s disease and healthy control subjects, recall of details (multiple choice recognition memory) after a 1-hour delay was compared for an emotionally arousing story versus a neutral story.
38 Both the control and Alzheimer’s disease groups had better memory for the emotional story, but the bvFTD group demonstrated a similar recall of details for both stories indicating compromise of the expected enhancement of memory conferred by emotional content. Structural imaging analysis (voxel-based morphometry) indicated that emotional enhancement of memory correlated with integrity of different regions in Alzheimer’s disease (hippocampus, parahippocampal, fusiform and frontal polar cortices) and bvFTD (orbitofrontal cortex, amygdala, insula).
Decreased volume of the insula has also been implicated in schizophrenia. A meta-analysis found medium effect sizes for reduced insular volume in schizophrenia compared with control subjects, with more substantial differences in the anterior than posterior insula.
39 However, there were not significant differences across the stage of illness, suggesting that volume reduction might be a predisposing risk factor. In contrast, an MRI study on 80 monozygotic twin pairs found reduced posterior insular gray matter volume in twins with schizophrenia compared with siblings without the illness.
40 Functionally, the insula seems to activate with positive symptoms. Impaired insular function may relate to an inability to differentiate the self from the nonself in patients with schizophrenia, thus driving delusional symptoms.
41–43 The insula has also been linked to hallucinations,
44 perhaps due to impaired salience network functioning.
45,46