Psychometric Properties of the Concise Associated Symptom Tracking Scale and Validation of Clinical Utility in the EMBARC Study
Abstract
Objective
Methods
Results
Conclusions
HIGHLIGHTS
METHODS
Study Design and Participants
EMBARC study
CO‐MED trial
Measurements
HAMD‐17
Quick Inventory of Depressive Symptomatology Self–Report (QIDS‐SR)
Concise Associated Symptom Tracking Scale Self‐Report
Altman Self‐Rating Mania Scale (ASRM)
Mood and Anxiety Symptoms Questionnaire (MASQ)
Anger attacks question
Adaptation of the CO‐MED Outcome Calculator
Statistical Analysis
RESULTS
| Itemb | CAST domain | Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | M | SD | ||
| 1. I feel anxious all the time | Anxiety | 22 | 8 | 49 | 17 | 62 | 21 | 120 | 41 | 39 | 13 | 3.36 | 1.14 |
| 2. I have been feeling really good lately | Mania | 117 | 40 | 120 | 41 | 40 | 14 | 13 | 4 | 2 | 1 | 1.83 | .86 |
| 3. I feel as if I am going to have a heart attack | Panic | 131 | 45 | 79 | 27 | 43 | 15 | 37 | 13 | 2 | 1 | 1.97 | 1.08 |
| 4. I wish people would just leave me alone | Irritability | 23 | 8 | 61 | 21 | 86 | 29 | 92 | 32 | 30 | 10 | 3.16 | 1.11 |
| 5. I have been having more trouble sleeping than usual | Insomnia | 18 | 6 | 54 | 19 | 56 | 19 | 111 | 38 | 53 | 18 | 3.43 | 1.16 |
| 6. I am feeling restless, as if I have to move constantly | Anxiety | 39 | 13 | 80 | 27 | 66 | 23 | 88 | 30 | 19 | 7 | 2.89 | 1.17 |
| 7. I suddenly feel very confident | Mania | 153 | 52 | 108 | 37 | 19 | 7 | 9 | 3 | 3 | 1 | 1.63 | .81 |
| 8. I am more talkative than normal | Mania | 119 | 41 | 124 | 43 | 26 | 9 | 18 | 6 | 5 | 2 | 1.85 | .94 |
| 9. I feel very uptight | Irritability | 28 | 10 | 51 | 18 | 57 | 20 | 122 | 42 | 33 | 11 | 3.28 | 1.17 |
| 10. I find myself saying or doing things without thinking | Irritability | 30 | 10 | 101 | 35 | 65 | 22 | 83 | 28 | 13 | 4 | 2.82 | 1.09 |
| 12. I can feel my heart racing | Panic | 62 | 21 | 96 | 33 | 50 | 17 | 75 | 26 | 9 | 3 | 2.56 | 1.17 |
| 13. Lately everything seems to be annoying me | Irritability | 12 | 4 | 44 | 15 | 611 | 21 | 130 | 45 | 45 | 15 | 3.52 | 1.05 |
| 14. I slept very little last night | Insomnia | 28 | 10 | 80 | 27 | 38 | 13 | 95 | 33 | 51 | 18 | 3.21 | 1.28 |
| 15. I cannot sit still | Anxiety | 47 | 16 | 109 | 37 | 68 | 23 | 54 | 19 | 14 | 5 | 2.59 | 1.11 |
| 16. I find people get on my nerves easily | Irritability | 7 | 2 | 57 | 20 | 50 | 17 | 121 | 41 | 57 | 20 | 3.56 | 1.09 |
| 17. I have been having lots of great ideas | Mania | 78 | 27 | 103 | 35 | 78 | 27 | 29 | 10 | 4 | 1 | 2.24 | 1.00 |
Validation of the CAST Scale's Psychometric Properties
Five‐domain structure
| Item/domain | Anxiety | Irritability | Mania | Insomnia | Panic |
|---|---|---|---|---|---|
| Standardized factor loadings | |||||
| I feel anxious all the time | .36 | ||||
| I have been feeling really good lately | .45 | ||||
| I feel as if I am going to have a heart attack | .61 | ||||
| I wish people would just leave me alone | .48 | ||||
| I have been having more trouble sleeping than usual | .62 | ||||
| I am feeling restless, as if I have to move constantly | .85 | ||||
| I suddenly feel very confident | .78 | ||||
| I am more talkative than normal | .70 | ||||
| I feel very uptight | .54 | ||||
| I find myself saying or doing things without thinking | .54 | ||||
| I can feel my heart racing | .93 | ||||
| Lately everything seems to be annoying me | .79 | ||||
| I slept very little last night | .68 | ||||
| I cannot sit still | .79 | ||||
| I find people get on my nerves easily | .85 | ||||
| I have been having lots of great ideas | .51 | ||||
| Pearson's correlation coefficient | |||||
| Anxiety | .1 | ||||
| Irritability | .43 | .1 | |||
| Mania | .39 | .13 | .1 | ||
| Insomnia | .30 | .08 | .07 | .1 | |
| Panic | .30 | .45 | .22 | .34 | .1 |
IRT analyses
| Threshold | |||||
|---|---|---|---|---|---|
| Item | Slope | 1 | 2 | 3 | 4 |
| Anxiety (3 items) | |||||
| 1. I feel anxious all the time | .73 | −3.75 | −1.72 | −.26 | 2.83 |
| 6. I am feeling restless, as if I have to move constantly | 3.50 | −1.24 | −.27 | .38 | 1.71 |
| 15. I cannot sit still | 2.73 | −1.18 | .10 | .87 | 2.00 |
| Irritability (5 items) | |||||
| 4. I wish people would just leave me alone | 1.19 | −2.62 | −1.02 | .32 | 2.32 |
| 9. I feel very uptight | 1.23 | −2.26 | −1.03 | −.14 | 2.10 |
| 10. I find myself saying or doing things without thinking | 1.22 | −2.19 | −.25 | .71 | 3.01 |
| 13. Lately everything seems to be annoying me | 2.76 | −2.08 | −1.01 | −.30 | 1.18 |
| 16. I find people get on my nerves easily | 4.49 | −2.17 | −.81 | −.29 | .90 |
| Mania (4 items) | |||||
| 2. I have been feeling really good lately | 1.26 | −.40 | 1.47 | 2.78 | 4.51 |
| 7. I suddenly feel very confident | 3.18 | .11 | 1.40 | 1.95 | 2.61 |
| 8. I am more talkative than normal | 2.30 | −.25 | 1.22 | 1.76 | 2.64 |
| 17. I have been having lots of great ideas | 1.34 | −.97 | .55 | 1.96 | 3.75 |
| Panic (2 items) | |||||
| 3. I feel as if I am going to have a heart attack | 5.92 | −.14 | .57 | 1.15 | 2.70 |
| 12. I can feel my heart racing | 1.71 | −1.11 | .15 | .78 | 2.77 |
| Insomnia (2 items) | |||||
| 5. I have been having more trouble sleeping than usual | 1.58 | −2.30 | −1.02 | −.26 | 1.33 |
| 14. I slept very little last night | 2.16 | −1.66 | −.44 | −.02 | 1.20 |
Internal consistency
Construct validity
| Domain | Anxiety | Irritability | Mania | Insomnia | Panic |
|---|---|---|---|---|---|
| QIDS‐SR | .22 | .24 | −.06 | .17 | .22 |
| 17‐item Hamilton Rating Scale for Depression (HAMD‐17) | .18 | .11 | −.09 | .34 | .18 |
| Irritability item of Anger Attack Questionnaire | .17 | .50 | .02 | −.03 | .12 |
| Irritability item of MASQ | .17 | .45 | −.03 | −.05 | .10 |
| Hamilton Rating Scale for Depression, anxiety scale | .24 | .21 | .05 | .12 | .22 |
| Altman Self‐Rating Mania Scale | .14 | .06 | .39 | .09 | .06 |
| QIDS‐SR, insomnia total | .09 | .04 | .05 | .38 | .12 |
| MASQ, general distress scale | .20 | .31 | −.24 | .04 | .17 |
| MASQ, anhedonic depression scale | −.02 | .13 | −.47 | .08 | .13 |
| MASQ, anxious arousal scale | .30 | .32 | .08 | .05 | .44 |
Validation of Clinical Utility
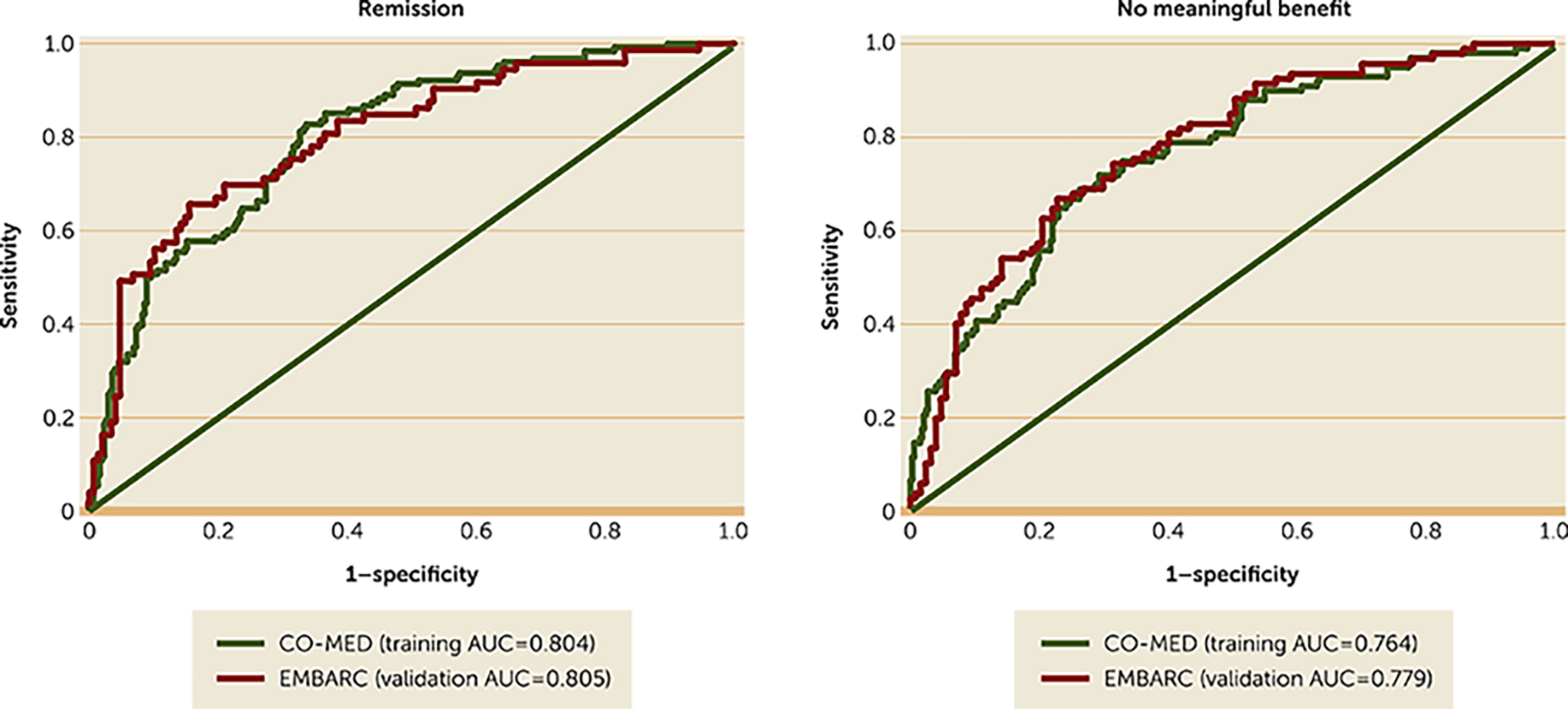
DISCUSSION
CONCLUSIONS
Footnotes
Supplementary Material
- View/Download
- 45.15 KB
References
REFERENCES
Information & Authors
Information
Published In
History
Keywords
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
