Depression is the leading cause of suicide in youth, and suicides among adolescents have risen by more than 50% between 2003 and 2017 (
1,
2,
3). More than 60% of adolescents do not have a single mental health encounter with either lay counselors or mental health clinicians in the year when they experience a major depressive episode (
4,
5). Thus, they do not receive first‐line evidence‐based treatments such as psychotherapies (
6,
7,
8), including cognitive, behavioral, and interpersonal (
9) or, when indicated, antidepressant medications (
10,
11). Appropriate treatment can improve health outcomes in youth (
12,
13).
Based on a controversial analysis of industry drug trials (
14) the Food and Drug Administration (FDA) issued a public health advisory in late 2003 that children and adolescents taking antidepressants were at increased risk of suicidal ideation and behavior (
15). The intent of the warnings was not to reduce care seeking, effective diagnosis, psychotherapy, and drug treatment, but rather to alert clinicians to monitor for suicidal ideation when starting treatment. Then, the FDA released a more serious “boxed warning” of this risk to be included in all antidepressant drug labeling effective in 2005. Finally, it extended the boxed warning to include young adults in 2007. This boxed warning continues to the present.
Unfortunately, the media published hundreds of alarming news stories that exaggerated the message from risk of suicidal ideation to risk of completed suicide (
16). This was reinforced by years of warnings of suicidality in television drug advertising and boxed warnings that have been associated not only with a chilling effect on drug treatment for depression, but also psychotherapy. Media exaggerations may have further stigmatized adolescents and young adults not to seek treatment for mood disorders and possibly changed prescribers' perceptions of the net benefits of antidepressants (
16,
17,
18).
Randomized trials are not feasible to examine the effects of drug safety warnings that are disseminated nationwide; therefore, the best research uses observational, quasi‐experimental designs. Several rigorous interrupted time‐series studies (
19) documented the unintended effects associated with the warnings. There were sudden, large declines in identification of depression among adolescents, young adults, and adults up to age 89 among 55 million enrollees in US health plans (
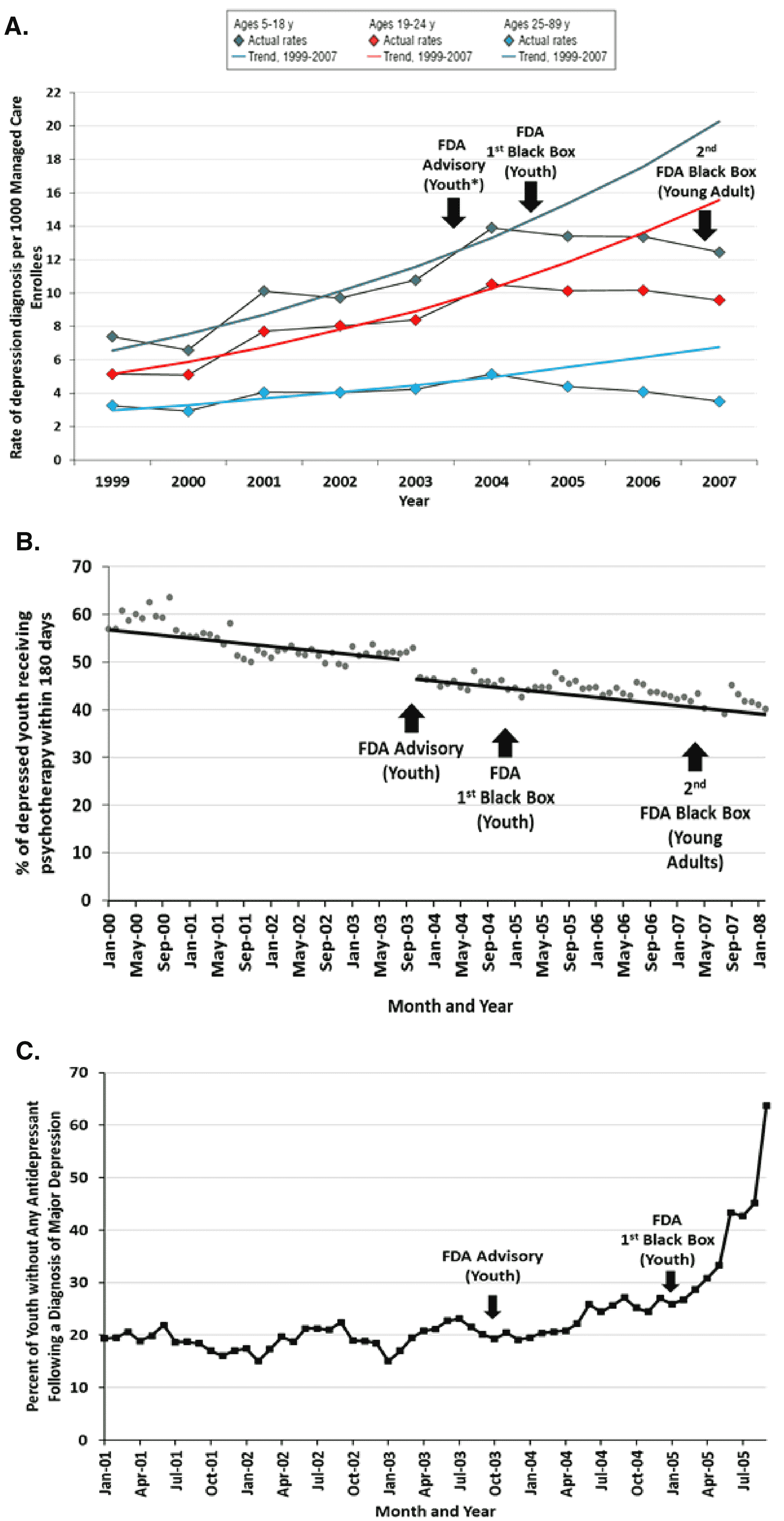
Figure 1A) (
20). The FDA warnings were not intended to reduce drug and nondrug treatment, but to increase monitoring for suicidal thoughts and behavior at the start of drug therapy (
21). However, psychotherapy among depressed patients of all ages continued a pre‐existing downward slope and a sudden, slight decline in level immediately after the warnings (
Figure 1B), suggesting a missed opportunity to discuss suicidal behavior (
15). In addition, controlling for a stable baseline trend, the FDA warnings were associated with a sudden, unintended threefold increase (from 20% to 60%) in the proportion of depressed youth without antidepressant treatment (
Figure 1C) (
20). Several studies (
22,
23,
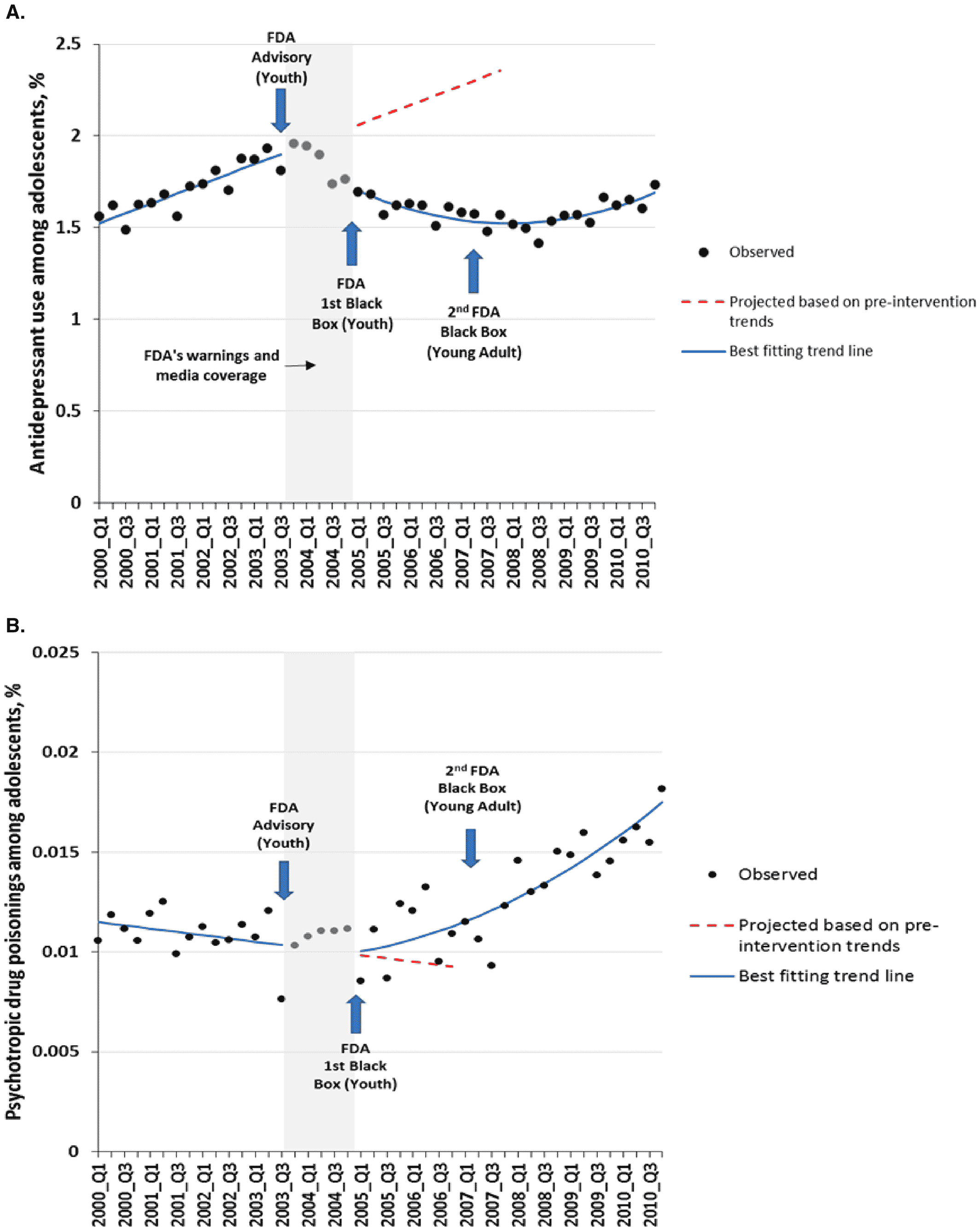
24) found 30%–50% declines in use of antidepressants among teens (
Figure 2A) and young adults (
22), including spillover effects to older adults not targeted by the warnings. Based on a national sample of health systems (
22) these declines in antidepressant use did not recover to earlier levels even 7 years after the first 2003 advisory (
Figure 2) (
22). Finally, a controlled, longitudinal study suggested a small increase after the warnings in psychotropic drug poisonings, a proxy for suicide attempts, in 11 US health plans (
Figure 2B) (
22).
The above identified unintended outcomes following the FDA warnings on depression care among young people represent possible mechanisms for changes in suicide deaths after the warnings. Sudden and significant changes in levels and slopes of these intermediate outcomes after the warnings, across large nationally representative populations, highlight the need for studies of their effects on rates of suicide. However, existing studies of suicide deaths lacked adequate sample size and sufficient longitudinal data (
22,
25). For example, at least three studies with only 1–2 years of post‐warnings data provided preliminary evidence of increases in suicide deaths after the warnings in the United States and Canada (
26,
27,
28). Here we report the first long‐term study to investigate whether suicide deaths in the United States increased among adolescents and young adults following the antidepressant warnings, and the accompanying declines in depression diagnosis and treatment already demonstrated in large quasi‐experimental studies.
Methods
We obtained population‐adjusted suicide death data between 1990 and 2017 from the WONDER Database, maintained by the Centers for Disease Control (CDC) and Prevention (
2). This database contains mortality counts based on death certificates for the US residents and population counts for all the US counties. These data are validated and are used in numerous CDC reports of trends in mortality in the United States (
29). We downloaded data on suicide deaths per 100,000 people in all states, based on cause of death by age group and year. CDC‐defined age groups in the database included adolescents (ages 10–19) and young adults (ages 20–24) (
2).
We used an interrupted times series design (
19), which can provide powerful evidence of policy effects when randomization is not possible (
30), because it measures the effects of interruptions in trends soon after policies and controls for secular trends in study outcomes. It does not resemble weak ecological analyses without pre‐policy baselines that are merely correlations of trends. The main assumption of this method is that extrapolating the pre‐policy level and trend correctly reflects the (counterfactual) outcome that would have occurred had the policy not happened. Time series of population suicide rates were divided into three segments: the 13‐year pre‐warning period (1990–2002), a transition period (2003 and 2004), and a 13‐year post‐FDA warning period (2005–2017) as the boxed warning officially started in 2005 for adolescents and then was extended to young adults in 2007. The first segment included rates of suicide death before the FDA warnings which define the counterfactual level and trend for measuring change in suicide death rates. The FDA released several initial advisories during late 2003 and 2004 so we displayed these data points but excluded this period from the statistical model. This approach evaluated the effects of the warnings at “full strength.” The post‐warning segment comprised rates of suicide death after the warnings. This method is consistent with our previous study (
22) of changes in antidepressant use and suicidality after the FDA warnings.
We used segmented regression models (
19) to estimate changes in the US suicide rates from the pre‐warning period to the follow‐up period for each age cohort. In all models, we controlled for baseline (counterfactual) trends. The models included terms to estimate changes in level and trend in suicide deaths after the warnings. Segmented regression models generally have a linear specification, but polynomial and nonlinear regression can be used where data exhibit nonlinear patterns. We included a quadratic term for the post‐warnings trend in our models because of observed nonlinearity. For parsimony, and consistent with prior research, we excluded nonsignificant (p≥0.05) time series terms in a stepwise fashion; exclusion of the nonsignificant terms did not change the coefficients on the remaining terms (
19). We controlled for all significant autocorrelation terms in the models because observations over time are correlated (
31). We graphed the observed and predicted suicide death rates from 1990 to 2017 based on the segmented models.
In addition, we estimated the size of cumulative effects on suicide deaths in the first 6 years immediately following the warnings (first 3 years since the 2007 warning expansion to young adults) by comparing the overall changes in outcome associated with the warnings with counterfactual estimates based on the pre‐warning trends (
22). Our cumulative effect estimates are conservative, using only 2005–2010 data, because confidence intervals are narrower (with smaller margins of error) sooner rather than later after the policy of interest. For the same reasons, inferences about possible effects of the warnings on immediate changes in suicide rates are stronger than any estimates years after the policy when other co‐interventions (confounders) could influence these outcomes. We conducted statistical analyses using SAS (version 9.3, SAS Institute, Cary, NC).
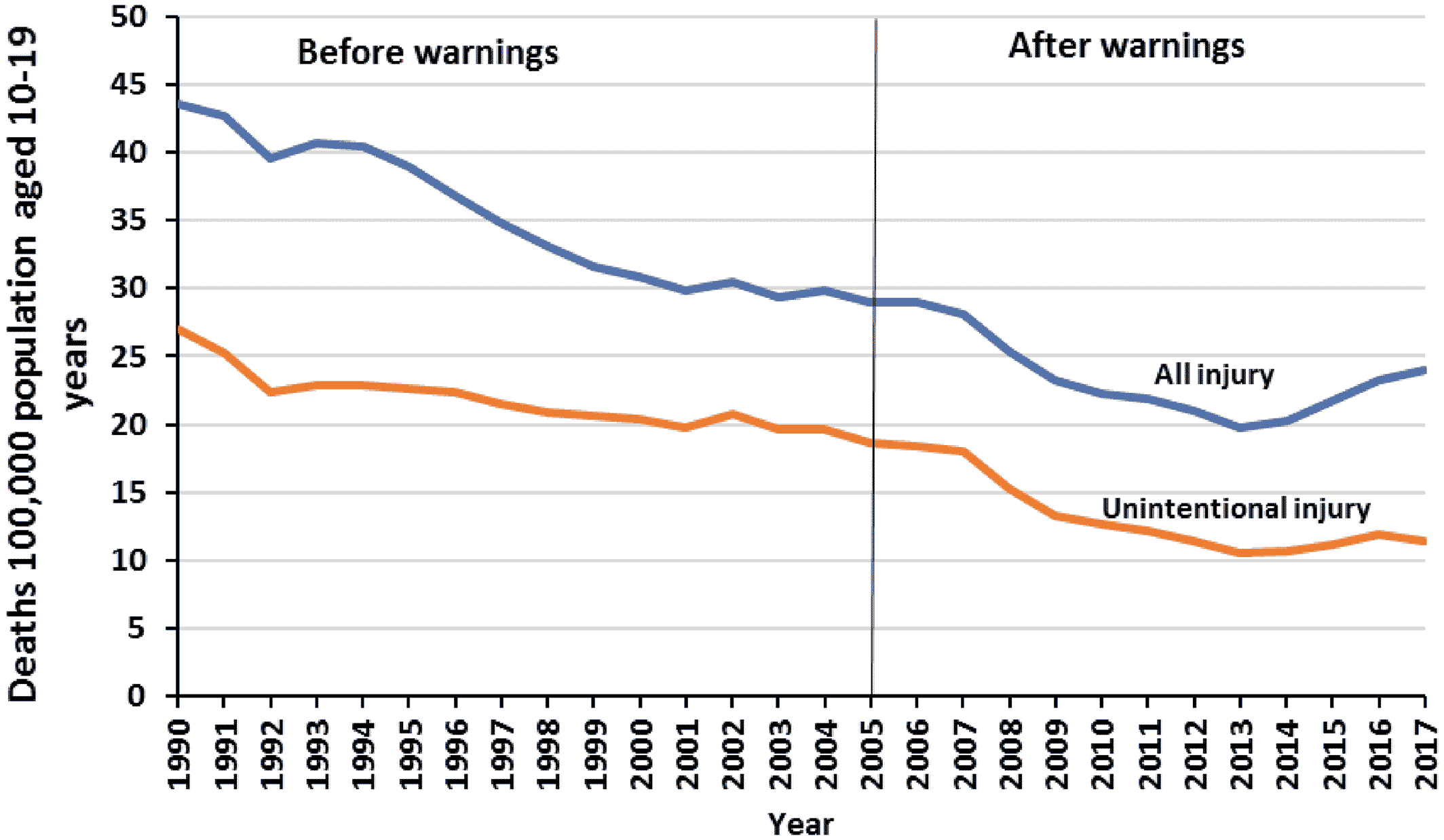
In a comparison analysis, we examined longitudinal trends in all injury deaths and unintentional (“accidental”) injury deaths because they should not be affected by the FDA warnings about antidepressants and suicidality risk. We obtained all injury deaths and unintentional (“accidental”) injury deaths for adolescents in the United States from 1990 to 2016 from WISQARS Fatal Injury Reports by the CDC (
32). The data are based on death certificates for the US residents. These data are validated and are used in numerous CDC reports of trends in mortality in the United States (
32).
We did not use older‐age groups, who were not the target population of the warnings, as formal controls for several reasons. First, both adults and elderly up to age 69 (
22) and age 79 (
15) also experienced reduced diagnoses and depression care after the FDA warnings (
Figure 1) (
15, 20, 22–24, 27). Second, they had several‐fold higher baseline suicide rates and different trends; this noncomparability in outcomes would bias effect estimates in difference‐in‐differences analyses (
33,
34). Third, there might be differential confounding by age. For example, the 2007–2009 recession, several years after the FDA boxed warning, was associated with higher risks of subsequent suicides among working adults and retirees, but generally not youth (
35,
36). Finally, abuse of opioids might have also increased suicides among adults, but not adolescents and those aged 20–24 (
37).
Results
Adolescents
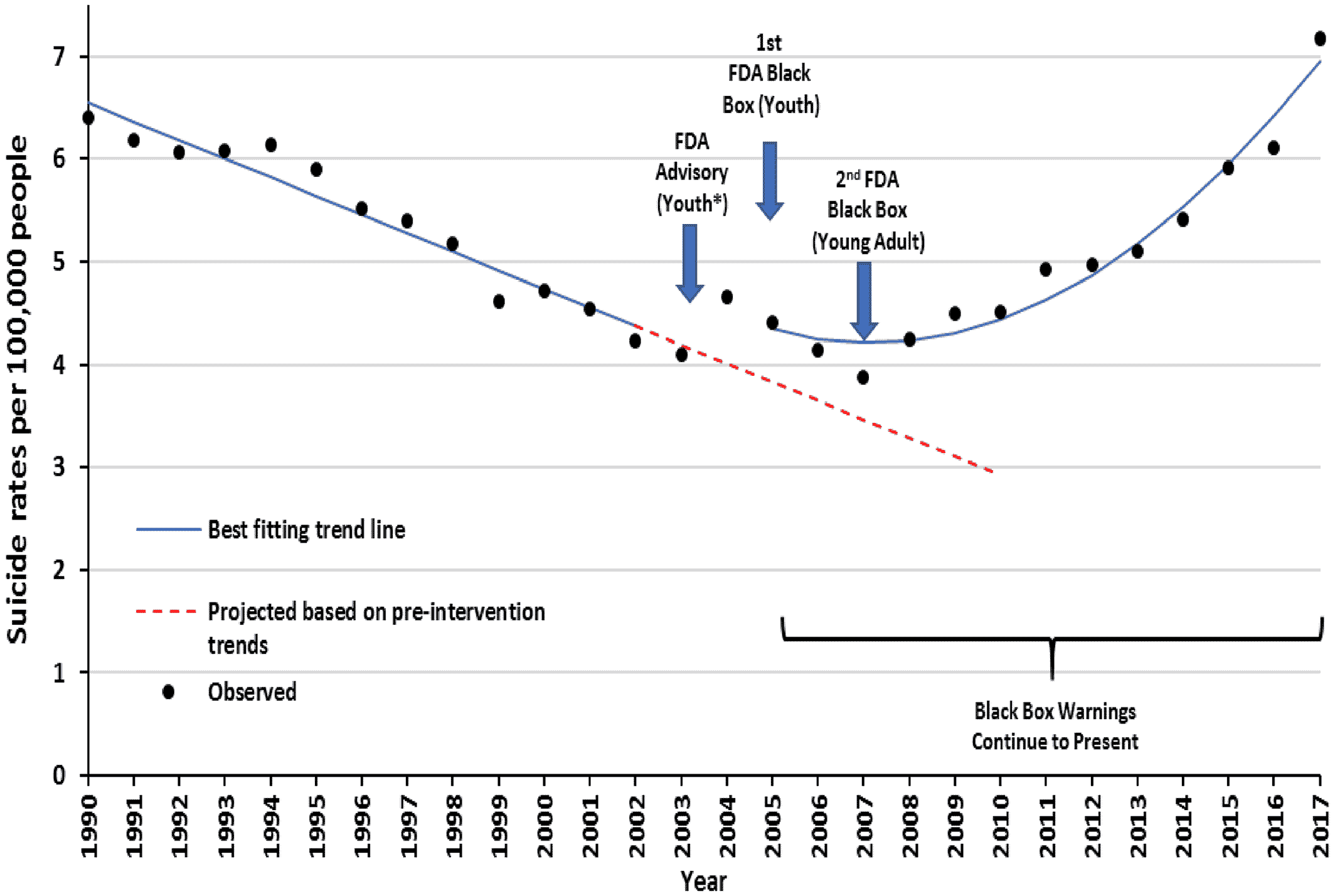
During the 13 years before the warnings, there was a significant downward trend in suicide deaths from 6.4 suicide deaths per 100,000 adolescents in 1990 to 4.2 in 2002 (−0.18 deaths per 100,000 adolescents per year; 95% confidence interval [95% CI]: −0.21, −0.15; p<0.001). After the 2005 FDA warnings and declines in depression care, this downward trend reversed; from 4.4 suicide deaths per 100,000 adolescents in 2005 to 7.2 in 2017 (
Figure 3). The increased level of suicide deaths deviated immediately from the baseline trend soon after the initial warnings. There was an immediate increase of 0.49 suicide deaths per 100,000 adolescents (95% CI: 0.12, 0.86) and a trend increase of 0.03 suicide deaths per 100,000 adolescents per year (95% CI: 0.026, 0.031). Comparing with counterfactual estimates based on pre‐warning trends, they suggest that the warnings may have caused 2365 excess suicide deaths in the United States from 2005 to 2010. The average annual population of adolescents aged 10–19 was 43 million.
Young Adults
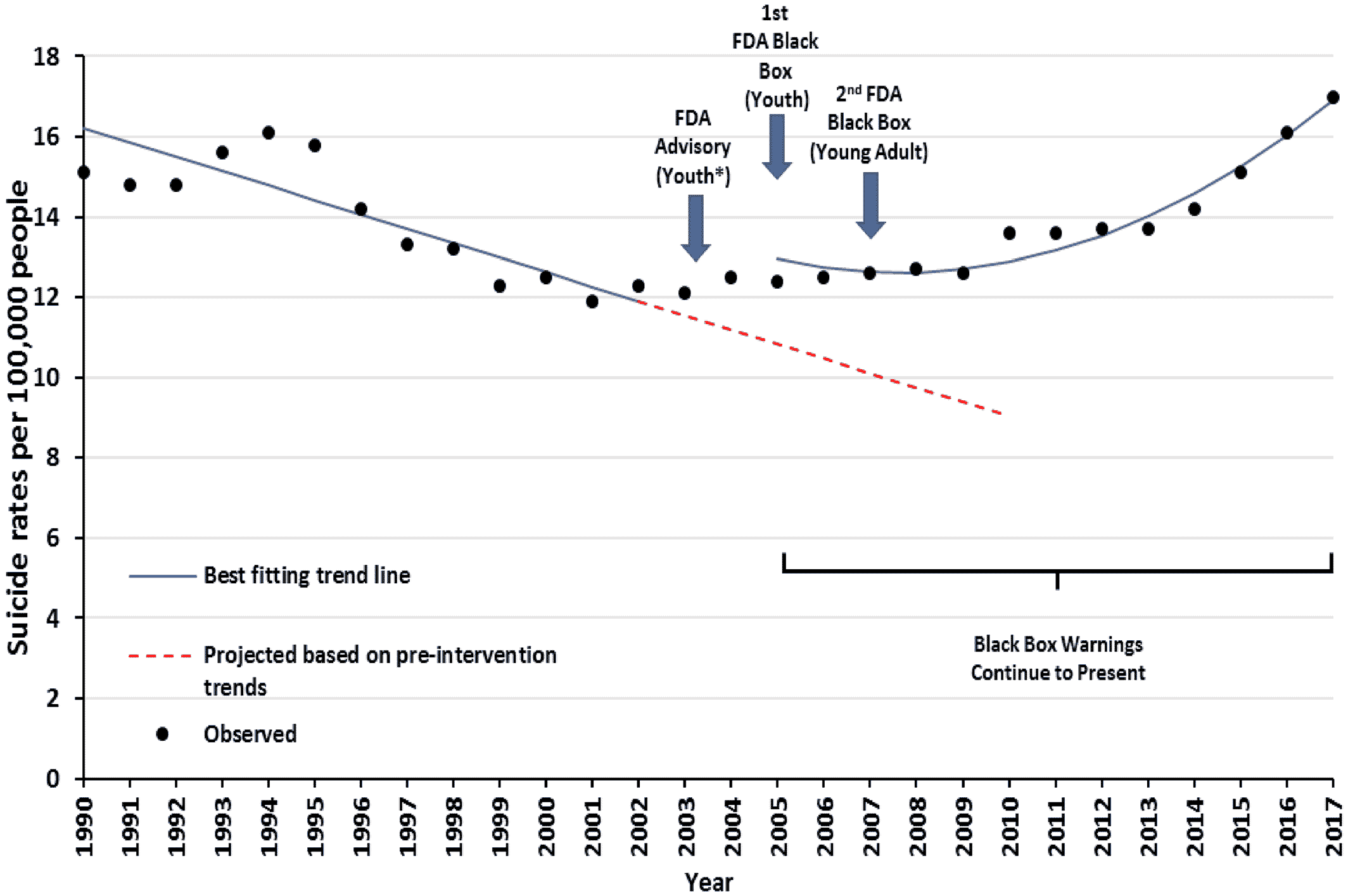
During the 13 years before the warnings, there was a significant downward trend in suicide deaths from 15.1 deaths per 100,000 young adults in 1990 to 12.3 in 2002 (−0.36 deaths per 100,000 young adults per year; 95% CI: −0.34, −0.28; p<0.001). After the 2005 FDA warnings and declines in depression care, this downward trend reversed; from 12.4 suicide deaths per 100,000 young adults in 2005 to 17.0 in 2017 (
Figure 4). There was an immediate increase of 2.07 suicide deaths per 100,000 young adults (95% CI: 1.04, 3.10), followed by a trend increase of 0.05 suicide deaths per 100,000 young adults per year (95% CI: 0.04, 0.06). Comparing with counterfactual estimates, these reflect 3593 excess suicide deaths from 2005 through 2010; the average yearly cohort of young adults was 21 million.
There was a downward trend in the comparison outcomes, all injury deaths and unintentional injury deaths among adolescents in the United States before the warnings; this decline continued relatively unchanged after the warnings (
Figure 5).
Discussion
In this nationwide study using 28 years of all US death certificates, controlling for a 13‐year stable downward trend in the pre‐warning period, we found that youth suicide deaths increased significantly after the FDA antidepressant warnings and reductions in depression care. Based on the annual size of these populations (averaged 43 million adolescents and 21 million young adults) in the United States, these may reflect 2365 excess suicide deaths among adolescents and 3593 among young adults from 2005 through 2010 (the first 6 years after the warnings). Other unintentional injury deaths did not increase after the warnings. While the factors influencing intentional and unintentional injuries may differ, these data add to the evidence of an association between the antidepressant warnings and increased suicide deaths.
A mechanism for this association is clear: The FDA antidepressant “warning,” accompanied by exaggerated media coverage, stigmatized depression care for young people, and fostered reluctance by providers to initiate antidepressant treatment. Thus, the warnings reduced diagnosis of major depression, and drug and nondrug mental health treatment.
Strong data support this association. Previous interrupted times series studies meeting the stringent design criteria of The Cochrane Collaboration (
38) described adverse effects of the warnings on care‐seeking, diagnosis, psychotherapy, medication use, and suicidality among both adolescents and young adults. These included: large reductions in trends of diagnosis of depression among youth, with spillover effects to adults (
15,
20,
22,
23,
24); a continued reduction in psychotherapy among depressed individuals of all ages (despite the warnings' major intent to
increase surveillance of young people's suicidal thinking and behavior) (
20); a 25% to 50% sudden decline in use of antidepressant medications among millions of adolescents and young adults both with and without depression diagnoses in low‐income Medicaid populations (
24) and in 11 US health plans (
22).
The warnings also reduced depression diagnosis and care among adults and the elderly (
15,
20,
22,
23,
24,
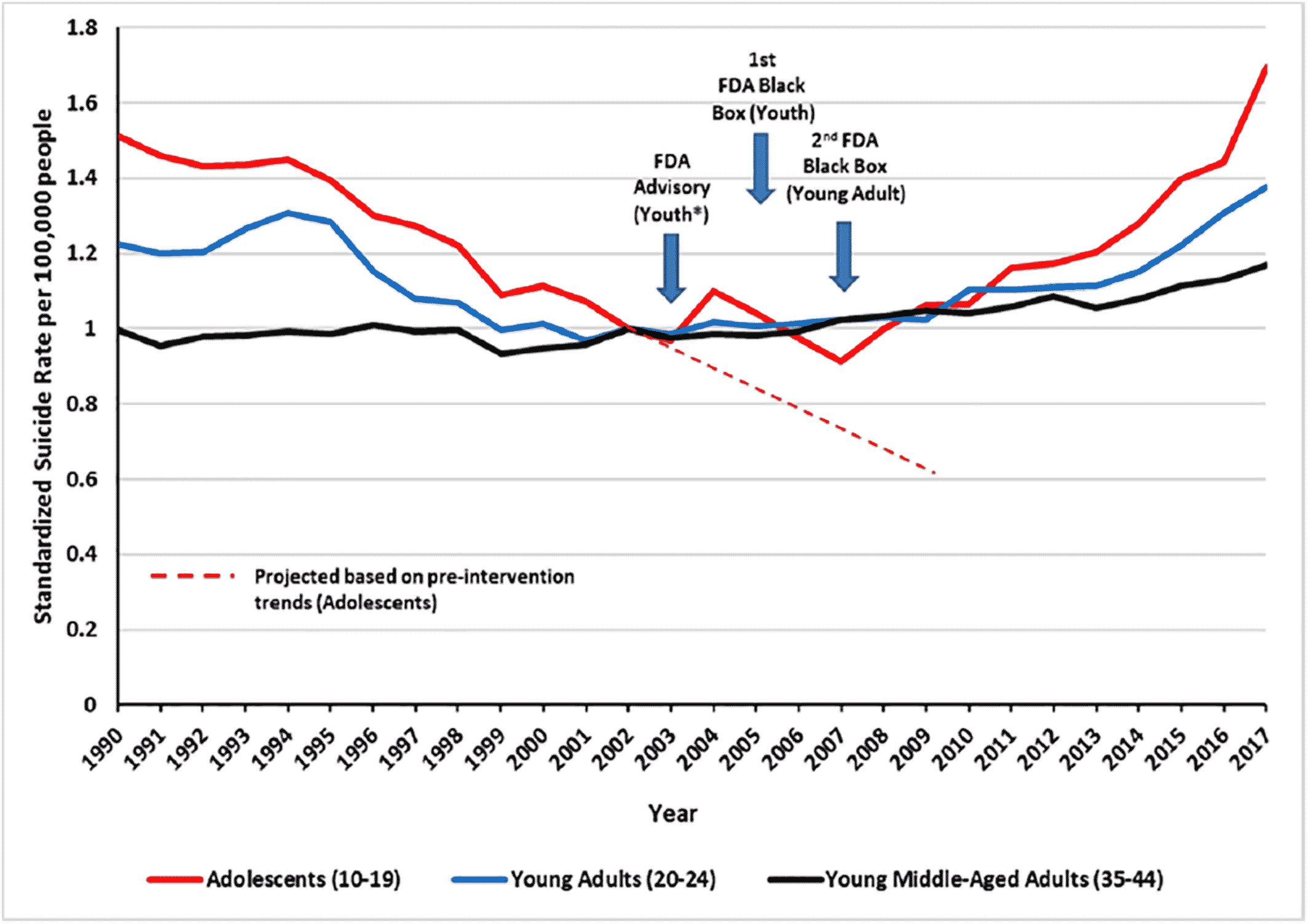
27). However, we did not attempt to statistically compare changes in suicides after the warnings among youth versus older adults (see Methods). Nevertheless, it is noteworthy that the increase in the trend of suicides among youth after the warnings exceeded those of younger middle‐aged adults (aged 35–44; Figure
A1). Thus, these graphical comparison group data support the results of our single group interrupted time series analysis, suggesting that the warnings contributed to increased suicide deaths among youth.
A single quasi‐experimental study cannot establish causality. However, the boxed warnings and associated reductions in depression care were further associated with a significant increase in suicide deaths among adolescents and young adults. This analysis indicates a pattern of unintended consequences that are significant enough to warrant action. There are currently no surveillance structures in place to identify unintended safety concerns associated with FDA drug warnings; however, the FDA is now working with Sentinel to develop a tool using these same interrupted time series methods to allow such monitoring (
39).
Critics of the hypotheses of increased risk of the antidepressant warnings have claimed that renewed increases in antidepressant use are the culprit, not the warnings' effects on all mental health care. However, studies have shown that 60%–70% of adolescents and young adults with major depression do not receive any mental health treatment from any clinician (
5) and that these high untreated rates were relatively unchanged from 2005 to 2014. While inappropriate use is certainly a concern, the major problem appears to be underuse, not overuse, of all mental health treatments leaving the increased risk from major depression unanswered.
There are several limitations of this quasi‐experimental study. The most important include any possible co‐interventions occurring around the time of the warnings that affect suicide rates. Some have hypothesized that the emergence of smart phones in the mid‐2000s may have exposed this group to new stressors: cyber‐bullying, status anxiety, and alienation, thus increasing suicide deaths in this group. However, this is unlikely to have explained the downward and upward trends before and after the warnings. The most definitive meta‐analysis of this relationship has now concluded that, although social media can have negative effects on self‐esteem and other outcomes, there are no or minuscule effects of smart phone use on suicide deaths in children and adolescents (
40,
41).
Another important co‐intervention is the last recession that occurred between 2007 and 2009 (
42). Data suggest that suicide deaths may have increased among adults, especially those who lost jobs (
35), and elderly people who experienced financial insecurity. This intervention occurred several years after the start of the antidepressant warning period. Few longitudinal studies have examined the effects of recessions on suicide among youth. While it is possible that some very vulnerable adolescents might be at increased risk of depression from experiencing large economic losses, recent data do not find an excess rate of suicide deaths in young people but do observe such an effect among adults (
36). Therefore, while this recession might have explained some individual deaths, it is unlikely to have caused adolescent and young adult population‐level shifts in suicide mortality that we observed in this study. The recession also does not explain the almost immediate reversal in the long decreasing trend in youth suicides from baseline occurring soon after the initial advisories and boxed warning (
Figures 3 and
4).
The opioid epidemic has also been identified as a possible risk factor for increased suicide deaths among adolescents (
3). However, this alternative explanation for our findings is unlikely. Rates of overdose deaths were rising steeply before the warnings when suicide deaths were declining sharply in our data. Conversely, and importantly, all deaths from drug abuse in adolescents declined by about 26% from 2007 (the time of the last boxed warning for antidepressants) to 2013 when suicide rates were increasing (
37). Adolescents are much less likely than older adults to abuse opioids (
37) which likely confound comparisons of the effects of the warnings on suicide risks across these age groups.
Furthermore, the ongoing, sharp increase in suicide rates after 2012, 5 to 7 years after the start of the warnings, are more difficult to attribute to only one policy. Additional economic, social, and medical factors may be contributing to the continued rise in youth suicide (
43).
Conclusion
In summary, this was the first study of the antidepressant warnings using 28 years of data from the US death certificates. We observed large increases in suicide deaths among adolescents and young adults following the widely publicized FDA antidepressant warnings, reductions in care‐seeking, diagnosis, and medication use, and increased suicide attempts coincident with the warnings. Other unintentional injury deaths, a comparison outcome, did not increase after the warnings. Furthermore, a significant majority of youth experiencing major depression do not receive any mental health care. These data support a formal re‐evaluation of the risks and benefits of the boxed antidepressant warnings, FDA's strongest type of communications about a drug or drug class. In this case, we recommend that the FDA err on the side of caution and consider replacing the boxed warning with less severe warnings that still communicate information on possible drug risks without endangering essential, first‐line treatments of depression in youth. Federal and local mental health agencies should undertake a nationwide public and media education campaign to increase the very low recognition and treatment of depression in adolescents and young adults.