Saccadic eye movements have been extensively used as a research tool to investigate the working functions of the brain in different neuropsychiatric diseases (
1). Among the numerous saccade paradigms, the anti‐saccade task (AST) makes it possible to study the mechanisms of voluntary saccade control. This task requires two processes executed in parallel: an inhibition of a reflexive eye movement towards a visual target (i.e., a pro‐saccade), and a volitional saccade directed away from the target to the (unmarked) mirror‐symmetrical location (i.e., an anti‐saccade) (
2). Such a response involves a complex neural circuitry including regions within the occipital, parietal and frontal cortices, superior colliculus, thalamus, striatum/basal ganglia, brainstem reticular formation, and cerebellum (
3). Given the dysregulation of prefrontal cortical/striatal and cerebellar circuits in subjects with attention‐deficit/hyperactivity disorder (ADHD) (
4), the AST may be used to reveal impairments in inhibitory control and executive function in such patients. Even though there are relatively few studies, it has been found in unmedicated adults with ADHD longer saccadic reaction times and more direction errors in the AST compared to healthy volunteers (
5,
6,
7,
8,
9).
Controlled studies demonstrated that the catecholamine reuptake inhibitor methylphenidate (MPH) is a safe and effective treatment for adults with ADHD (
10). However, owing to the heterogeneity of the disorder (i.e., etiological, clinical, neurobehavioral, and neurobiological), almost one‐third of adults with ADHD show no or little improvements with MPH treatment (
11). It has been shown that, even at low dose, MPH induces procognitive effect via stimulation of dopamine (DA) D1 receptors and
α2‐adrenoreceptors in the prefrontal cortex (
12). Consistent with this mechanism of action, the AST performances are enhanced following acute and/or chronic MPH administration in children and in adolescents (
13,
14). However, in adults, such studies are lacking, although our group recently reported that a single low dose of MPH (10 mg orally) administered in drug‐naive patients normalized the AST reaction times and direction errors (
9).
Due to the need for more appropriate intervention strategies in the treatment of ADHD, the primary aim of our study was to examine whether the AST performance after the first MPH‐dose in adults newly diagnosed with ADHD could be useful for predicting the long‐term response to MPH.
Discussion
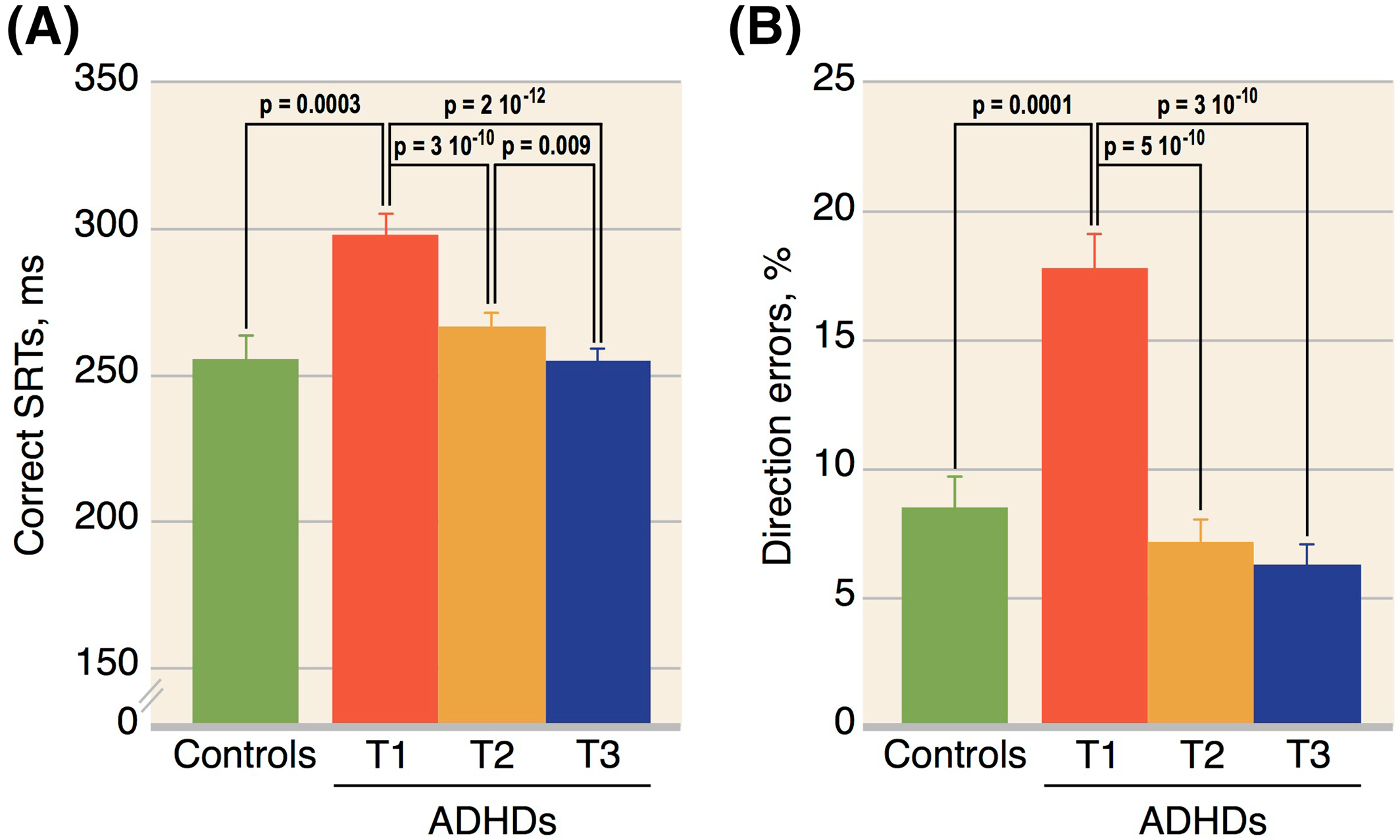
The main findings of our pilot study are as follows: (1) never‐medicated adults with ADHD make more direction errors and have longer SRTs in the AST than HCs; (2) MPH administration, either acute or chronic, normalizes the AST performances; and (3) a low percentage of direction errors after the first MPH‐dose (≤10%) is associated with subsequent remission.
Our results of increased direction error rates, at baseline, are consistent with all previous AST published studies in unmedicated ADHD adults (
5,
6,
7,
8,
9). Moreover, delayed SRTs and normal rates of express saccades have also been previously described (
5,
7,
8,
9), although it has been sometimes reported normal SRTs (
6) or increased anticipatory saccades (
7,
9). These few discrepancies may come from methodological differences, and more particularly from the heterogeneity of the populations studied. In previous studies, patients were also very often treated chronically with stimulating drugs despite a short period of withdrawal before the oculomotor tests (
5,
6,
7,
8). Thus, one cannot rule out a remanent effect of stimulant treatment on the AST performances—or, conversely, a “rebound effect” given upregulation of DA transporter availability during long‐term treatment (
21).
It is commonly agreed that SRTs reflect the programming stage duration in the saccadic system and that directions errors triggered at regular latencies are produced when the automated signals override the voluntary signals (
2). From a pathophysiological viewpoint, slower SRTs and increased direction errors in ADHD could be attributable to impairments of motor planning and response inhibition (
22), although it is likely that multiple factors and pathways contribute to the suppression process. Hence, in line with the hypothesis of frontostriatal hypoactivation in ADHD (
23,
24), difficulty in suppressing the automatic pro‐saccade may be accounted for the impaired function of the frontal lobes (especially the dorsolateral prefrontal cortex) mediating top‐down inhibition of saccade neurons (
3). The generation of volitional saccades, on the other hand, requires activation of the neural saccade network, including the superior colliculus, the frontal eye fields (whose activity has been correlated with the anti‐saccade reaction time), the supplementary eye fields (which play an essential role in the execution of voluntary saccades), and the anterior cingulate cortex (activated in preparing saccades) (
3). Hakvoot Schwerdtfeger et al. (
8) hypothesized that extended SRTs and increased direction error rates in ADHD reflect mainly a poor preparation for inhibiting automatic pro‐saccades, rather than a deficit in the saccade execution. Given the heterogeneity of ADHD, it is conceivable that deficits in the AST performance could also be related to hyper‐responsivity of the superior colliculus (
25)—although in our ADHDs the proportion of anticipatory/express saccades was rather low—, and/or reduced vigilance/arousal levels (
26) given adults with ADHD show frequently poor sleep quality and excessive daytime sleepiness ((
27), for review).
Confirming our previous findings (
9), the first dose of the psychostimulant MPH administered at a low dose (10 mg) in drug‐naive ADHDs normalizes SRTs and direction errors. It has been suggested that acute MPH induced‐DA and noradrenaline changes in corticostriatal‐cerebellar systems (associated with top‐down executive control improvements) (
28), and ventral striatal‐limbic systems (associated with bottom‐up motivation and reward improvements) (
29), mediate enhancements in the AST performance. Consequently, by increasing catecholaminergic activity, especially in the prefrontal cortex even at a low dose (
12,
30), MPH may restore voluntary inhibitory control on saccades. Moreover, by elevating the arousal level (
31), MPH may also increase the inhibitory power of the prefrontal cortex‐basal ganglia circuitry (
5).
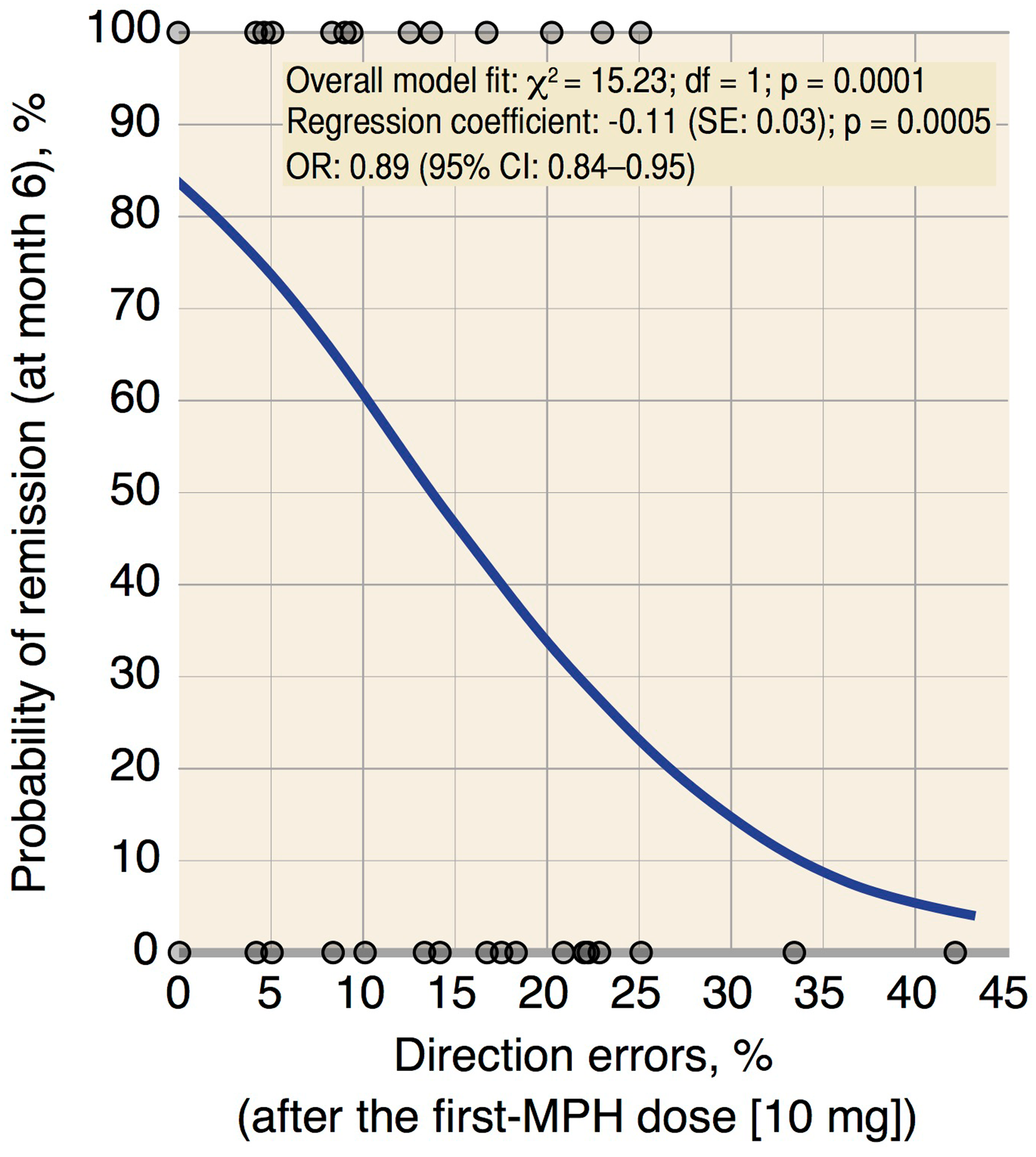
In accordance with the existing literature (
11), about two‐thirds of our ADHDs experienced clinical remission after 6 months of MPH treatment. Interestingly, the AST measures at endpoint are quite comparable with those obtained with acute MPH administration, despite a modest but significant reduction in SRTs possibly reflecting slight improvements on the neural saccade network secondary to chronic MPH administration. It is noteworthy that the initial AST performances (at baseline) are not associated with subsequent treatment outcomes, while after the first MPH‐dose low direction error percentages (i.e., ≤10%), but not SRTs, are associated with clinical remission, even adjusting for age, sex, weight, and severity of symptoms at baseline. As revealed using multivariate logistic regression analysis, ADHDs with direction errors ≤10% after the first MPH‐dose are 85% more likely to become remitters than those with direction errors >10%. The biological bases for the inter‐variability of clinical responses to MPH in adults are not clearly elucidated, but they probably involve differences in the catecholaminergic tone (
32,
33). Thus, it can be presumed that stimulant therapy would be efficient by correcting the baseline hypocatecholaminergic condition (
34). This is in line with evidence that enhanced catecholamine transmission in response to MPH administration mediates frontal activation (
12,
30,
35) and regularizes the arousal level (
31). In this frame, the percentage of direction errors after the first MPH‐dose, by indirectly assessing the catecholaminergic system responsivity, may provide a reliable clue for predicting subsequent response to MPH treatment. This is further supported by the fact that there is a relationship between the direction error rates after the first MPH‐dose and the evolution of ADHD symptoms following 6 months of MPH treatment.
The strengths of this present study are that we evaluated never‐MPH medicated adults with ADHD (avoiding therefore previous treatment bias), and that we repeated the AST three times in the same subjects (at baseline, after acute administration of a standard dose of MPH, and 6 months after chronic administration of MPH, titrated to a dose producing maximal benefits without adverse side effects). To the best of our knowledge, this is the first study to examine long‐term effects of MPH on the AST performances in adults with ADHD. Our findings are also buttressed by the large sample that was treated with MPH (
n = 97). However, some limitations should be mentioned. The first one might be the lack of placebo use as a comparative treatment. However, the aim of our study was not to demonstrate the effectiveness of MPH in ADHD but to evaluate the oculomotor correlates of clinical response. As previously discussed (
9), it seems unlikely that improvement in the AST performances with MPH may be attributed to a placebo effect or a learning effect. Second, we did not evaluate the effects of MPH on the AST performances in HCs. To date, only one study (
36) performed in healthy subjects reported that anti‐saccades latency and errors are unaffected following acute administration of 20 mg of MPH. Third, as in the vast majority of studies, we did not measure plasma concentration of MPH, and consequently no link can be drawn between plasma MPH concentration and improvement in the AST performances. Finally, although our findings appear to be statistically robust, they must be considered preliminary until replicated in a larger population.
In conclusion, this pilot study provides evidence that MPH administration, either acute or chronic, can reverse the AST abnormalities in adults with ADHD. Interestingly, a low rate of direction errors triggered at regular latencies after the first MPH‐dose is independently associated with favorable clinical outcome; this could potentially provide early therapeutic decision help in a clinical setting. Further controlled studies in a wider population are needed to confirm the value of this prognostic oculomotor marker.