The pandemic and the recent murders of George Floyd, Ahmaud Arbery, and others have shined a spotlight on health disparities and systemic racism in this country. Since Africans were first brought to this continent against their will by the Spanish in 1526 (
1), government and societally sanctioned atrocities against African descendants have continued (
2,
3). Historical trauma and ongoing systemic racism has a toll, not just on the psyche of African Americans, but on their physical health as well (
4,
5,
6,
7). It is well established that personal experiences of trauma, adversity, and discrimination can “get under the skin” and increase risk for a whole host of negative psychiatric (
8,
9,
10) and medical health problems (
11,
12,
13,
14,
15,
16,
17) through stress, brain, epigenetic, and immune system mechanisms (
7,
18,
19). Emerging data now also suggests that adversities and traumas in one generation, can be passed down to affect the health and well‐being of future generations. Through transgenerational epigenetic mechanisms, grandchildren and great grandchildren can be negatively impacted by ancestral traumas – even when they have not been directly exposed to any harm themselves (
20,
21).
Systemic racism is associated with experiences of persistent discrimination and elevated rates of exposure to inadequate nutrition and toxicants (e.g., lead, air population) that impact an individual's health and well‐being. While economic success may prevent offspring exposure to inadequate nutrition and harmful toxicants, it cannot eliminate all experiences of discrimination. Enduring health disparities, therefore, are likely to be due to both personal and ancestral adversities.
This paper reviews key concepts in transgenerational epigenetic inheritance research, select studies examining the role of epigenetic mechanisms in transmitting the impact of ancestral stress and trauma, poor nutrition, and toxicant exposure across generations, and factors that can mitigate the effects of these experiences. Culturally adapted interventions that address historical trauma and systemic racism are also briefly discussed.
While it is beyond the scope of this manuscript to elaborate on the societal factors that perpetuate adversity and impact the health and well‐being of African Americans in the United States, the authors acknowledge that trauma did not end for this population with the Emancipation Proclamation. It continued through Jim Crow, lynchings, (
3) and redlining practices (
32,
33), and persists today via the well‐documented systemic biases in the criminal justice system (
34). As depicted in
Figure 1 (
21), the current discriminatory criminal justice system policies not only negatively impact the person behind bars – but the entire family. Children whose fathers are incarcerated tend to have minimal contact with their parent while he is in prison (
32,
33), are apt to experience food insecurity (
35), and are more likely to live in neighborhoods that are socioeconomically disadvantaged (
36). These neighborhoods are associated with poorer quality schools, a concentration of environmental hazards, including lead and air pollution (
37), fewer safe outdoor spaces for children to play, and higher rates of crime and community violence (
38). And as we know from countless examples (
39), even when African American families transcend the cycle of disadvantage propagated by government and societal policies and practices, privilege does not guarantee one can keep their loved ones safe.
TRANSGENERATIONAL EPIGENETIC INHERITANCE: KEY CONCEPTS
Genetic inheritance occurs through the DNA passed from parents to their offspring through the gametes, with the DNA from the male carried in the sperm, and the DNA from the female is carried in the egg. When the egg and the sperm unite, they form a single cell which will have to multiply and make all the different cell types required for life. Every cell in the body has the same DNA, but different genes are turned on in different cells, making, for example, a neuron different than a cardiac muscle cell. The development of each different cell type is programmed through
epigenetic mechanisms – chemical modifications to the DNA that change its three‐dimensional shape and the likelihood of a given gene product being turned on or off. Blood, kidney, and brain cells begin to develop by the fifth week in utero, with normal development proceeding through a predictable order, although the full range of molecular mechanisms responsible for normal embryonic and fetal development are not fully known (
40).
Epigenetic mechanisms are also one of the ways experiences of trauma and adversity get “under the skin.” Methylation – the addition of a carbon atom with three hydrogen atoms to DNA – is known to shut off genes when added to the beginning of the gene sequence, and to increase expression when occurring elsewhere on the gene. One of the most highly replicated findings in the field of epigenetics research is that experiences of early life stress can lead to DNA methylation of the glucocorticoid receptor (GR) gene. First noted in 2004, by 2016 this finding had been replicated in 40 independent investigations, 13 animal and 27 human studies. The glucocorticoid receptor helps to turn off the stress response, and methylation of the GR gene is associated with reduced number of glucocorticoid receptors and heightened stress reactivity (
41).
Whether or not these environmentally induced, epigenetic modifications can be inherited and transmitted across generations is an active area of research. Studies of transgenerational epigenetic inheritance are hard to execute in humans as it is difficult to obtain multigenerational cohorts and exclude psychosocial (e.g., poverty) and cultural (e.g., racism) confounders that may lead to common epigenetic, behavioral, and health outcomes across generations (
29,
42). However, as reviewed in the following section, a growing body of animal research suggests the effects of traumatic stress and other negative exposures can be transmitted to subsequent generations through epigenetic mechanisms (
21,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55).
For the environmentally induced epigenetic modifications to be inherited across non‐successive generations, they must be contained in the germline – the sperm or the egg – as these are the only two cells used to create subsequent life. Transgenerational epigenetic inheritance of traumatic stress and other negative exposures (e.g., lead) requires: 1) epigenetic modifications in the exposed animal/individual be present in the germline (e.g., sperm, egg); 2) epigenetic modifications in the exposed animal/individual be causally linked to the negative outcomes associated with the exposure; 3) the negative outcomes associated with the exposure be evident in subsequent generations with no history of exposure; and 4) the presence of the negative outcomes in the subsequent generations be causally linked to the epigenetic modifications initiated in the first exposed generation.
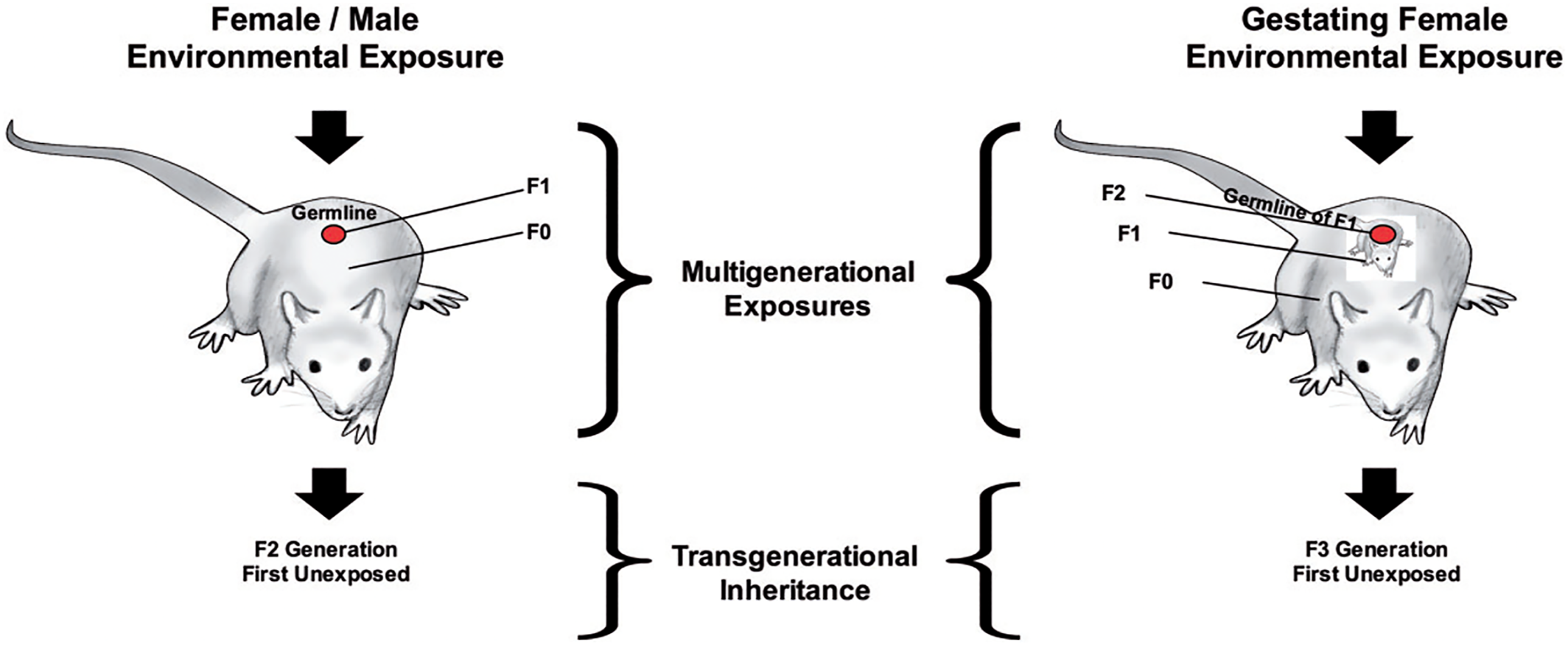
Figure 2 (
56), depicts the experimental paradigm used to investigate transgenerational epigenetic inheritance in animals (
56). The male or female that has the initial negative environmental exposure is labeled the F0 generation. As the impact of exposure can lead to epigenetic modifications to the germline (e.g., sperm, egg) of the F0 generation that creates the F1 generation, the F1 generation is considered ‘exposed’ as well. Transgenerational inheritance cannot be examined until the F2 generation. If as depicted on the right side of the diagram, the female is pregnant at the time of exposure, she (F0) is exposed; her developing baby (F1) is exposed, and its germline and the subsequent offspring (F2) is also considered exposed. The F3 generation (e.g., great grand offspring) would then be the first unexposed offspring in which transgenerational inheritance could be examined.
The three forms of epigenetic modifications linked most frequently to transgenerational inheritance include: DNA methylation, as discussed previously; histone modifications; and the action of non‐coding RNA molecules (ncRNA) (
57). Histones are the proteins that act as spools to wind DNA, and chemical modifications to histones can also affect gene regulation. While RNA molecules are best known for their role in coding proteins; ncRNAs can also act as epigenetic factors and impact gene regulation (
56).
Although evidence of transgenerational inheritance has been reported through the female germline (
58), most transgenerational inheritance studies have focused on examining epigenetic factors in sperm due to the relative ease of obtaining large numbers of sperm cells for analyses. Eggs cannot be readily obtained or acquired in large enough quantity for traditional molecular analysis, and there are other factors that confound the testing of oocyte transmission. Currently more research is needed using evolving single‐cell analytic techniques and cross‐fostering designs to fully elucidate the role of the female germline (e.g., eggs) in epigenetic inheritance (
56).
Because sperm develop behind a protective barrier (
59), there was skepticism about the capacity for environmental exposures to elicit epigenetic modifications in sperm. Recently three independent teams were able to demonstrate that extracellular vesicles could transmit information about environmental stress and other adverse exposures to sperm, leading to epigenetic modifications that could be transmitted intergenerationally (
59,
60,
61).
Skepticism about transgenerational epigenetic inheritance also flourished as DNA methylation and histone epigenetic marks are known to be erased after fertilization so the cells of the evolving embryo can be totipotent – capable of transforming into all the different cell types required for life (
21,
30,
31). It has since been established that erasure and reprogramming is not complete, (
53,
56,
59,
62,
63) and as discussed in the following section, DNA methylation, ncRNAs, and histone epigenetic marks appear to be involved in facilitating experience‐dependent transgenerational inheritance.
TRANSGENERATIONAL EPIGENETIC INHERITANCE: RESEARCH REVIEW
Rodent studies have reported epigenetic modifications in the germline (e.g., sperm) which have been causally linked to the inheritance of negative outcomes associated with stress, (
50,
51,
59,
64,
65,
66) a high fat diet (
67,
68,
69), and multiple chemical exposures (
44,
46,
47,
53,
57,
58,
70,
71,
72,
73). Many of these studies, however, only provide evidence of multigenerational transmission (see
Figure 2). Select studies that provide evidence of true transgenerational inheritance – with the impact of ancestral exposures evident in subsequent generations with no history of exposure – are reviewed here.
Dias and Ressler used fear conditioning to study the transgenerational transmission of traumatic stress (
64). They subjected F0 mice to odor fear conditioning before conception by pairing an odor, an innocuous stimulus, with a shock, so that with time the odor alone elicited fear. They then found that subsequently conceived F1 (e.g., children/offspring) and F2 (e.g., grandchildren/grand offspring) generations had an increased behavioral sensitivity (e.g., startle response) to the F0‐conditioned odor, but not to other odors, despite no prior exposure to the odor or shocks. F0 mice subjected to fear conditioning and their F1 offspring were also found to have epigenetic marks in their sperm in the odorant receptor gene (
Olfr151). Enhanced behavioral response to the F0‐conditioned odor was also associated with alterations in the olfactory epithelium and olfactory bulb in the F1 and F2 generation offspring of F0 fear conditioned mice. These same neuroanatomical alterations were also observed in odor naïve mice generated using in vitro fertilization (IVF) with sperm from the F0 fear conditioned mice, suggesting the neuroanatomical changes to the olfactory system were transmitted through the male germline (e.g., sperm). Due to animal quarantine issues, however, behavioral studies could not be conducted with the IVF‐generated offspring.
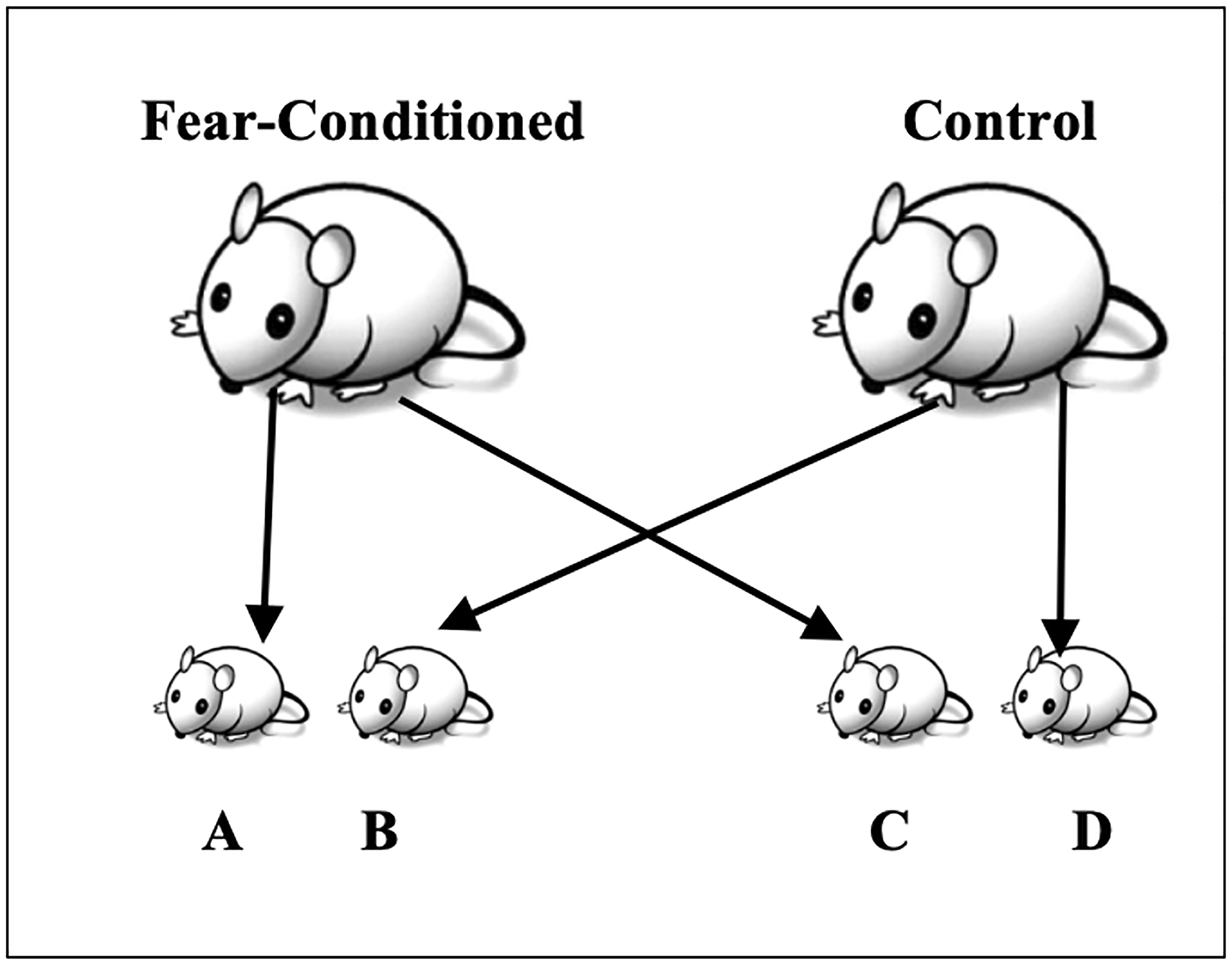
To determine if behavioral sensitivity to the conditioned stimuli could be transmitted via the female, Dias and Ressler conducted a cross‐fostering study using the design depicted in
Figure 3 (
64). Sexually naive female mice were conditioned with the odor (i.e., fear conditioned) or left in their home cage (control). They were then mated with odor‐naive males. Immediately after birthing their offspring were then divided into the following groups: (A) offspring of the fear‐conditioned mothers raised by the fear‐conditioned mothers; (B) offspring of the control mothers raised by the fear‐conditioned mothers; (C) offspring of the fear‐conditioned mothers raised by the control mothers; and (D) offspring of the control mothers raised by the control mothers. The females were only exposed to the odor conditioning before mating, and never while pregnant, precluding in utero exposure. Offspring of the fear‐conditioned mice, whether raised by fear‐conditioned mothers, or raised by the control mothers, exhibited increased behavioral sensitivity to the F0‐conditioned odor, suggesting conditioned fear responses can be transmitted via the female germline as well.
Yao and colleagues examined the impact of ancestral and multigenerational stress on maternal weight gain, gestational length, maternal blood glucose levels, and offspring weight in a four generation study (
66). Pregnant rats in the first (F0) generation were exposed to stress from gestational days 12 to 18. In this study, “stress” involved being put in a confined environment that limited movement and a brief forced swim challenge. The pregnant female offspring (F1) and grand female offspring (F2) of the F0 moms were either exposed to stress or left undisturbed (e.g., non‐stressed). Outcomes were examined in each generation, including in great grand offspring (F3). Stress reduced maternal weight gain in the F0 cohort and each successive generation, decreased gestational length beginning in the F1 cohort, and increased maternal blood glucose levels by the F2 cohort. Decreased offspring weight was evident by the F1 cohort and greatest in the F3 offspring of transgenerationally stressed mothers. As depicted in
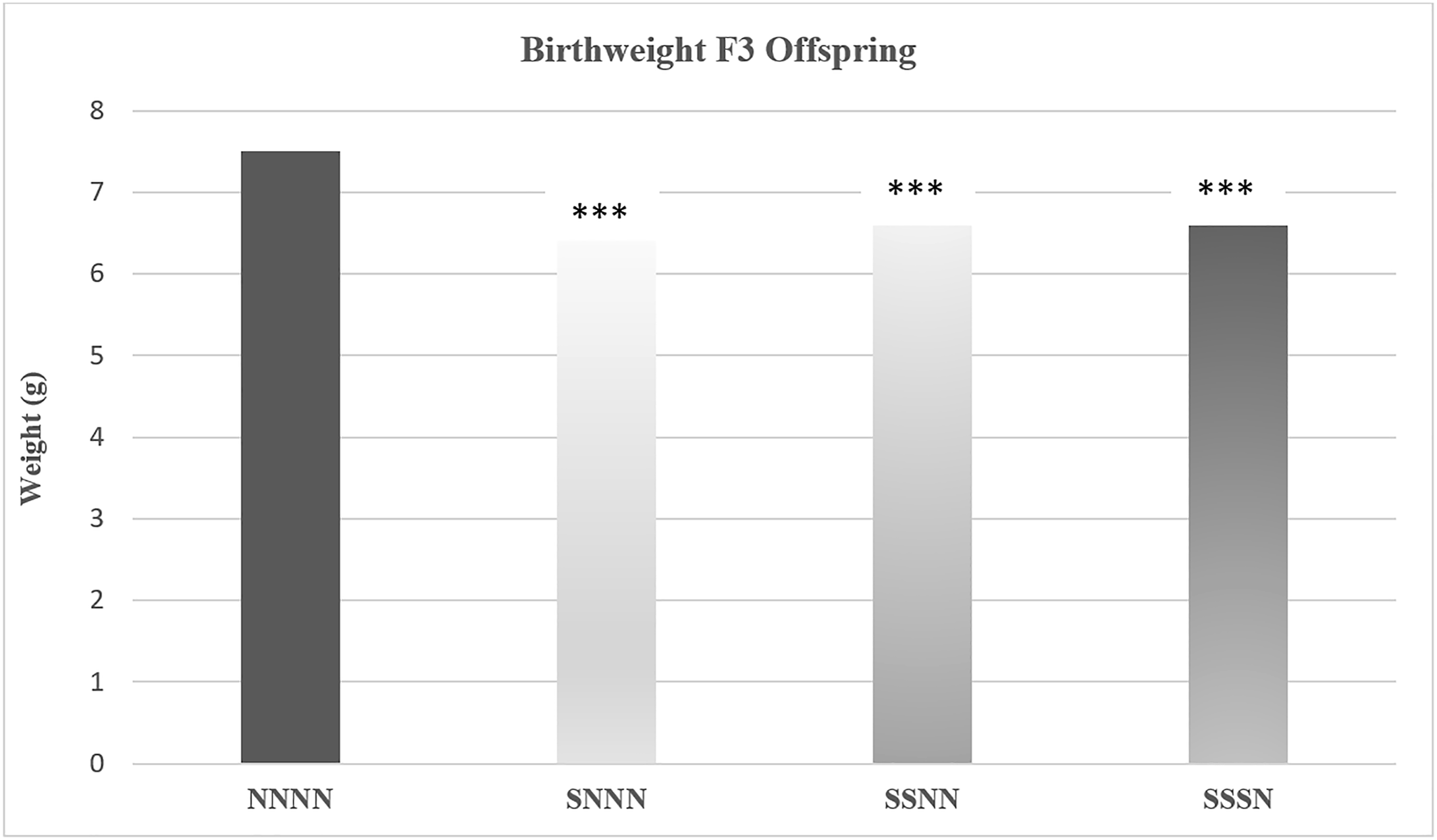
Figure 4 (
66), transgenerational and multigenerational prenatal stress resulted in low birth weight among F3 offspring. In the diagram NNNN indicates there was no stress across the four generations; SNNN indicates only F0 transgenerational stress, and SSNN and SSSN are indicative of multigenerational stress.
In addition to the impact on birth weight, offspring of prenatally, multigenerationally, and transgenerationally stressed mothers were reported to exhibit developmental delays. Yao and colleagues also conducted brain frontal cortex, uterus, and placenta ncRNA and gene expression analyses in F0‐N, F0‐S, and F2‐SSS animals, with results in F2 stressed animals demonstrating that a multigenerational history of prenatal stress is associated with changes in genes implicated in brain plasticity (e.g., miR‐200 family genes), parturition/childbirth (e.g., Zeb2), and preterm birth (e.g., miR‐181a) (
66). The documentation of an impact of transgenerational and multigenerational stress on preterm birth is particularly interesting given racial disparities in rates of preterm birth, and recent findings that adequate prenatal care does not reduce racial disparities, with African American women who engage in adequate prenatal care still at elevated risk for preterm birth (
74).
de Castro Barbosa and colleagues showed that a high‐fat diet could reprogram the epigenome of sperm and transgenerationally affect metabolism in the offspring (
68). In this study, F0 male rats were fed either a high‐fat or normal chow‐diet for 12 weeks and then mated to normal chow‐fed females to create F1 and F2 generation offspring. Sperm were isolated from F0 and F1 males. The F0 male rats fed the high‐fat diet had increased body weight and impaired glucose tolerance. The F1 (e.g., offspring) and F2 (e.g., grand offspring) offspring of the F0 males fed the high‐fat diet had reduced birth‐weight when compared to the offspring of chow‐fed F0 males; with low birth‐weight a documented risk factor for obesity and type 2 diabetes (
75,
76). F0 male rats fed the high‐fat diet and their F1 male offspring had common sperm DNA methylation and small ncRNA expression signatures – with several of the epigenetic sites identified in genes implicated in the regulation of glucose homeostasis, insulin sensitivity, and a predisposition to Type 2 diabetes (e.g., let‐7c) (
68). Consistent with these data demonstrating the role of a high‐fat diet in programming the epigenome of sperm to affect the metabolism of the offspring, Grandjean and colleagues showed that microinjection of either testis or sperm ncRNA of male mice fed a high‐fat diet into naive one‐cell embryos lead to the establishment of the high‐fat diet‐induced metabolic phenotype (e.g., insulin resistance, impaired glucose tolerance) in the resulting progenies, whereas ncRNAs prepared from healthy controls did not (
69).
To the best of our knowledge, no rodent transgenerational studies have examined the impact of lead exposure, a major public health hazard for African American urban children, with profound and well‐characterized developmental and behavioral implications across the lifespan (
77). Meyer and colleagues used zebrafish to study the transgenerational repercussions of lead exposure (
77). F0 embryos were exposed for 24 h to waterborne lead. The F0 generation zebrafish were then raised to adulthood and F1 and F2 generation offspring, who had no direct lead exposure, were then studied. The dosage of lead exposure used in this investigation was previously found to generate learning impairments in zebrafish, and similar learning impairments were found to be present in the F2 offspring of F0 lead exposed zebrafish. RNA was extracted from the brains of the F2 grand offspring of control and lead‐exposed F0 zebrafish. Significant expression differences were found in 648 genes, with path analyses revealing altered expression in genes involved in brain development (e.g., synaptic function and plasticity, neurogenesis), endocrine homeostasis, and epigenetic processes – genes which may be involved in lead‐induced neurobehavioral deficits and/or their inheritance. These data provide an initial step in demonstrating the potential transgenerational health effects of lead exposure (
77).
The observation by Dias and Ressler that in vitro fertilization with sperm from F0 fear conditioned mice generated offspring that had the same olfactory perception brain changes that were observed in the F1 and F2 generation offspring of F0 fear conditioned mice provides strong support that transgenerational epigenetic inheritance is transmitted through the male germline (e.g., sperm) (
64). Comparable support for the role of the male germline in epigenetic inheritance has been reported in multiple other studies using similar methodology across fewer generations. For example, Gapp and colleagues injected sperm ncRNAs from males subjected to an early stress paradigm into eggs and produced offspring with the behavioral and metabolic alterations associated with their early stress experimental paradigm (
65); Rodgers and colleagues generated offspring with patterns of stress dysregulation observed in mice subjected to their chronic stress paradigm by microinjecting a zygote with sperm ncRNAs altered by the chronic stress protocol (
52); and Chan and colleagues produced offspring with neurodevelopment and stress reactivity indices similar to their stress‐treated animals using assisted reproductive technology with sperm from naïve adult male mice that was incubated with extracellular vesicles from stress‐treated animals (
59).
The biological relevance of the genes regulated by the epigenetic marks identified in the studies of transgenerational epigenetic inheritance also provides compelling support for the role of these mechanisms in the transmission of experience‐dependent traits and health problems. To review, the F0 mice subjected to fear conditioning using an odor and their F1 offspring were found to have epigenetic marks in their sperm in a gene critical to olfactory perception (
64). A multigenerational history of prenatal stress which promoted reduced gestational length and developmental delays in the offspring was associated with changes in genes implicated in brain plasticity, parturition/childbirth, and preterm birth (
66). F0 male rats fed the high‐fat diet and their F1 male offspring had epigenetic alterations in genes implicated in the regulation of glucose homeostasis, insulin sensitivity, and a predisposition to Type 2 diabetes (
68); and the F2 offspring of lead‐exposed F0 zebrafish had significant expression differences in genes involved in brain development (e.g., synaptic function and plasticity, neurogenesis) which may be relevant in understanding lead‐induced cognitive and neurobehavioral deficits.
There is also emerging data suggesting the relevance of this research for understanding the transgenerational transmission of the effects of adversity and other negative exposures in human cohorts. Beyond the epidemiological studies which suggest parental exposure to trauma and stress, inadequate nutrition, and toxicants can impact the health of descendants across several generations (
29,
30,
31), several investigators have documented the presence of the epigenetic marks noted in the rodents in human samples. For example, alterations in the ncRNAs (e.g., miR‐16, miR‐37) reported in the sperm of mice subjected to maternal separation have been observed in the serum of children aged 7–12 years of age who experienced paternal loss and maternal separation, the serum of adult men aged 18–25 years of age who likewise experienced paternal loss and maternal separation at a young age, and the sperm of adult men aged 21–50 years of age who experienced two or more significant traumatic events in childhood (
65,
78). In another study, male adults with a history of early life stress exhibited reduction in a ncRNA (e.g., miRNA‐434) in sperm that was also reported to be altered in a mouse model of early life stress (
79). The finding of alterations in this particular ncRNA in the sperm of adults with histories of early life stress was also replicated in an independent sample (
78). Experiences of recurrent stress in healthy adult males was also found to be associated with changes in ncRNAs detected in sperm that were identical to the ncRNA changes reported in a mouse study of chronic stress in adult animals (
80).
While more work is needed to fully elucidate the mechanisms by which experience can alter the epigenome and impact health and developmental trajectories in subsequent generations, the accumulating body of animal research is quite compelling. The role of the female germline in transgenerational epigenetic inheritance requires further investigation, but the cross‐fostering study by Dias and Ressler (
64), and the four‐generation pregnancy stress investigation by Yao and colleagues (
66), suggest a role for the female germline in epigenetic inheritance (
56). Elucidation of the molecular mechanisms involved in environmentally induced epigenetic transgenerational inheritance is essential to fully understand disease etiology (
53), and has important implications for the development of novel prevention and treatment interventions to mitigate the negative impact of deleterious ancestral exposures. The relevance of this preclinical research in understanding human disease, however, requires carefully designed multigenerational studies.
FACTORS THAT MITIGATE THE EFFECTS OF HISTORICAL AND PERSONAL TRAUMA
The transgenerational negative effects demonstrated in the animal studies reviewed in the prior section can be prevented. Animal studies showing ways to mitigate the deleterious effects of the various exposures on the F0 and subsequent generations are highlighted in this section, with parallel and other promising human interventions also discussed.
In the initial study by Dias and Ressler (
64), the pairing of an odor with a shock (e.g., fear conditioning) was used to model the effect of traumatic stress in the F0 and subsequent generations. In a follow‐up experiment, the same procedures were used, but a subset of the animals were provided “treatment” to eliminate the elicitation of fear by the odor (
81). “Treatment” was comprised of extinction training – the gradual elimination of the conditioned response (e.g., fear when presented with the odor) by repeat presentation of the odor without any shocks.
Animals that were initially conditioned to fear the odor and then provided “treatment” stopped exhibiting fear when exposed to the odor. Their offspring (F1) also did not show increased behavioral sensitivity (e.g., startle response) to the F0‐conditioned odor. In addition, the epigenetic changes observed in the sperm in the gene critical to olfactory perception was only evident in the mice conditioned to fear the odor and not provided any “treatment”; the mice that received extinction training (e.g., treatment) did not have these epigenetic marks in their sperm. It appears “treatment” can prevent the transgenerational transmission of the negative effects associated with ancestral traumatic stress.
Extinction training is at the core of all evidence‐based psychotherapeutic approaches for treating Posttraumatic Stress Disorder (PTSD) in children, adolescents, and adults (e.g., Exposure Therapy, Trauma‐Focused Cognitive Behavioral Therapy), with talking about and visualizing the traumatic events (e.g., repeat exposure) paired with relaxation training and cognitive processing (
82,
83,
84).
These interventions are highly effective in diverse populations for a broad range of traumatic experiences (e.
g.,
sexual abuse,
intimate partner violence,
community violence,
traumatic loss of a loved one).
de Castro Barbosa and colleagues showed that a high‐fat diet could reprogram the epigenome of sperm and transgenerationally affect metabolism in the offspring (
68). An independent group using a similar mouse model demonstrated that diet or exercise interventions for 8 weeks in obese males prior to conception prevented the development of metabolic problems (e.g., insulin sensitivity, excess adipose tissue) in the offspring (
85). Paternal diet and exercise also prevented changes to sperm ncRNAs. We are not aware of comparable multigenerational obesity interventions in humans, but these animal studies suggest preconception diet and exercise programs may help to break the transmission of obesity and associated negative health outcomes (e.g., diabetes, cardiovascular disease, cancer, and premature mortality).
There is,
however,
a plethora of data that suggests adopting a healthy lifestyle can diminish an individual's risk for obesity and these other health problems.
Reducing intake of red meat (
86,
87),
consuming plant protein over animal protein (
88),
having regular portions of fruit (
86,
87),
eating foods rich in antioxidants or taking antioxidant supplements (
89,
90),
refraining from excessive alcohol use (
86,
87),
avoiding smoking (
87,
91),
doing physical exercise, (
87,
91,
92)
and engaging in mindfulness‐based stress reduction activities (
89,
90)
are all associated with longevity and reduced risk of these stress‐related health problems. As food deserts – areas lacking in affordable healthy foods – are concentrated in minority neighborhoods (
93), federal efforts to enhance access to quality foods through the Healthy Food Financing Initiative, which provides incentives for healthy food retailers to open stores in areas lacking access to nutritious fresh food, may be an important first step in addressing the obesity epidemic in the Black community (
94). Available data, however, suggests that access alone is not always sufficient to improve residents' diets (
94,
95,
96), indicating additional targeted interventions are required.
Many of the other deleterious transgenerational effects reviewed in the prior section were found to be prevented when, after the initial negative exposures, the F0 cohort was provided enrichment experiences (e.g., living in enhanced spaces that included toys to provide rich social, physical, and sensory experiences). For example,
female mice subjected to prenatal stress who were subsequently provided enrichment experiences did not experience preterm birth and their offspring did not show any developmental delays (
97,
98), male mice subjected to early stress who were provided enrichment experiences did not exhibit the sperm epigenetic changes associated with the early stress paradigm and there was no transmission of any stress‐related behaviors to their offspring, (
99) and providing enrichment experiences to female rats exposed to lead while gestating prevented the development of lead exposure‐related deficits in the cognitive performance of their offspring (
100).
Studies in children,
adolescents,
and young adults also suggest a role for enrichment experiences in mitigating the impact of personal and ancestral traumas. The Harlem Children's Zone programs and the Carolina Abecedarian Project, which provided educational enrichment programs to low income African American youth, have been found to promote resilience and a range of long‐term positive developmental outcomes which are sustained across time and generations (
101,
102,
103). Participation in team sports is also associated with resilience, specifically, reduced mental health problems among youth with histories of significant childhood adversities (
104). The Stanford Medical Youth Science Program, which provides academic enrichment in the sciences and college admissions support to very low‐income minority high school students, most with poor academic preparation, has also been associated with very positive outcomes (
103). Of the more than 400 youth who completed the program, 99% have been admitted to college, 81% earned a four‐year college degree, and among four‐year college graduates, 52% are attending or have graduated from medical or graduate school.
CULTURAL ADAPTATIONS OF PREVENTION AND INTERVENTION PROGRAMS
The Strong African American Families (SAAF) intervention is an evidence‐based intervention developed for 11‐year‐old youth which is designed to enhance the parent‐child relationship and address issues unique to African American youth (e.g., racial socialization, racism). The developers of SAAF have also created programs for older youth, and programs to enhance parenting relationships (
105,
106,
107,
108,
109). SAAF was initially developed for youth from low‐income families from disadvantaged neighborhoods in rural Georgia; however, it is currently being implemented in urban communities around the nation (
110,
111), including 24 social services agencies in Harlem (
112). SAAF consists of seven consecutive 2.5‐h weekly family group meetings held at community facilities, with separate skill‐building curricula for youths and their primary caregivers. The caregiver sessions emphasize positive parenting skills, including the consistent provision of instrumental and emotional support, high levels of monitoring and control, adaptive racial socialization strategies, and methods for communicating about sex and alcohol use. Youth sessions focus on forming goals for the future and making plans to attain them, resistance efficacy skills, and adaptive behaviors to use when encountering racism. At SAAF meetings, families eat a meal together and then divide into small parent and child discussion groups. For the final hour of each session, the caregivers and youth reunite for a two‐generation group meeting.
The SAAF program has been associated with positive outcomes on child behavioral problems, health risk behaviors, health problems, and number of physiological indices. Specifically, SAAF participation has been associated with decreased rates of conduct problems in youth two years after the intervention (
113); reduced rates of smoking (
114), drinking (
115), drug use, (
116) and risky sexual behaviors (
117) in late adolescence and early adulthood; and reduced risk of obesity (
118) and prediabetes in young adulthood (
119). In the latter study, adverse childhood experiences were not associated with risk for prediabetes in young adults who participated in the SAAF intervention, but among the youth in the control intervention, each additional experience of adversity was associated with a 37% increase in risk for prediabetes (
119). In terms of physiological indices, the SAAF intervention was associated with reducing the impact of family risk factors on stress system (e.g., adrenaline, norepinephrine) (
120), inflammation (
121), and epigenetic (
122,
123) markers. The parenting‐focused SAAF intervention was also associated with diminishing the impact of poverty on hippocampal and amygdala brain volumes measured in adulthood—key brain regions affected by stress (
124). The investigators note their findings are consistent with a possible role for supportive parenting in brain development, and appear to suggest a strategy for narrowing social disparities (
124). The programs focus on positive racial socialization and equipping youth to deal with racism likely also greatly contributes to the success of the intervention.
Over the past two decades there have been over a dozen meta‐analyses examining the effectiveness of culturally adapted psychotherapeutic interventions (
125). Positive findings have been reported for individual, group, and family culturally adapted treatments (
126), with culturally adapted interventions associated with better outcomes when compared to the same intervention without the adaptations (
g = 0.52, medium effect size). In a large meta‐analysis with nearly 14,000 participants, culturally adapted interventions had a 4.68 times greater likelihood of producing remission from psychopathology than the non‐adapted version of the intervention (
125). The success of cultural adaptations of prevention and intervention programs in producing positive treatment outcomes and mitigating the impact of personal adversity highlights the importance of utilizing these approaches to address health disparities and promote resilience and recovery.