Substance use disorders (SUDs) are associated with significant morbidity and mortality in the adolescent population. Recent estimates indicate approximately 8.5% or 2.2 million adolescents, aged 12–17 in the U.S. met DSM‐5 criteria for a SUD in the past year, based on individual self‐report (
1). Early exposure to substances can have detrimental effects, increasing the risk of developing SUDs, mood disorders, and the transition to other forms of substance use later in life (
2,
3,
4). Adolescents who use substances are at higher risk of victimization, bullying, and high‐risk sexual behaviors (
5,
6,
7). Higher rates of suicidality and overdose are also observed in this patient population (
8,
9). Timely recognition and diagnosis of SUDs is necessary to decrease the risk of future health consequences.
There is considerable variation in the frequency and use of evidence‐based screening tools among clinicians targeting adolescent substance use, often influenced by barriers such as limited time, training, and familiarity with standardization (
10,
11,
12). Proper SUD diagnosis is critical as positive screens are often insufficient in eliciting appropriate intervention (
12). The majority of adolescents who need specialty substance use treatment do not receive it, even after an overdose event (
1,
13). Those who are able to be enrolled in care often require efficient treatment planning given the scarcity of adolescent‐tailored resources (
14). Processes that streamline the receipt of accurate SUD diagnosis to treatment placement are needed to connect adolescents with timely care.
The Global Appraisal of Individual Needs‐Initial (GAIN‐I) is an assessment tool used to diagnose and develop treatment plans for mental health (MH) disorders and SUDs using patient self‐report for adolescents and adults (
15). The GAIN‐Quick Version 3 (GAIN‐Q3) was developed from the original, lengthier version to screen for substance use, MH and associated problems in a shorter period of time, while assessing an individual's level of current functioning or impairment (
16). The Global Appraisal of Individual Needs‐Quick Version 4 (GAIN‐Q4) is an adaptation of the GAIN‐Q3 and integrates additional validated items from the GAIN‐I to further address diagnostic criteria for SUDs and co‐occurring MH disorders. The aim of this study is to investigate the utility of the GAIN‐Q4 as a diagnostic tool for SUDs and co‐occurring MH disorders in adolescents ages 12–17.
DISCUSSION
This study evaluates the reliability of the GAIN‐Q4 as a diagnostic tool for SUDs in adolescents. Results demonstrate the ability of the GAIN‐Q4 to diagnose any SUD with good sensitivity (89%), excellent specificity (93%), substantial kappa (0.80) and excellent percent agreement (90%). The GAIN‐Q4's ability to diagnose specific SUDs (cannabis, alcohol, stimulant, opioid and other) in this population is highlighted by excellent sensitivity values (>90%) across all diagnoses and good percent agreements (>80%) excluding alcohol use disorder (68%). Results from this study also emphasize the GAIN‐Q4's ability to detect problems associated with co‐occurring diagnoses in adolescents with excellent sensitivity (97%).
The GAIN‐Q4 performed similarly to the well‐established GAIN‐I measure (
16,
20,
21,
22), and provides a single instrument that can quickly and reliably diagnose SUDs and co‐occurring disorders in adolescents. Failure to recognize and treat SUDs in adolescence increases the risk of negative consequences throughout adulthood. Earlier onset of substance use is associated with higher rates of future health problems and risk behaviors, including development of mood disorders and transition to use of other types of substances with more severe consequences, such as opioids (
3,
27). Current screening instruments for substance use in adolescents are limited by their inability to provide diagnoses and subsequently encourage appropriate treatment intervention (
28,
29). Results of this study indicate that the GAIN‐Q4 can offer accurate diagnosis of any SUD in a timely manner, thus providing a valuable clinical instrument for use in this population.
The GAIN‐Q4 performed well when identifying adolescents with specific SUDs, displayed by its high sensitivities (>90%) across all substances analyzed in this study. The agreement between the GAIN‐Q4 and its parental counterpart for diagnosing specific SUDs was fair based on Cohen's kappa (>0.3) and good based on percent agreement (>80%) except for alcohol use. This could be explained by the GAIN‐Q4's overestimation of specific SUD prevalence compared to the GAIN‐I presented in
Table 1. We suspect that part of the challenge was the high prevalence of alcohol use (potentially in more frequent but lower amounts) with other drug use and disorders. But this study was not designed to address this hypothesis. From a clinical perspective, identification of any concerning substance use is critical, so individuals can quickly initiate therapy and treatment that can then be tailored for specific or multiple SUDs. Additionally, substance use items may cross over frequently due to the high prevalence of co‐morbid SUDs. The results of this study demonstrate that the GAIN‐Q4 can quickly recognize SUD patterns in adolescents to efficiently facilitate treatment initiation.
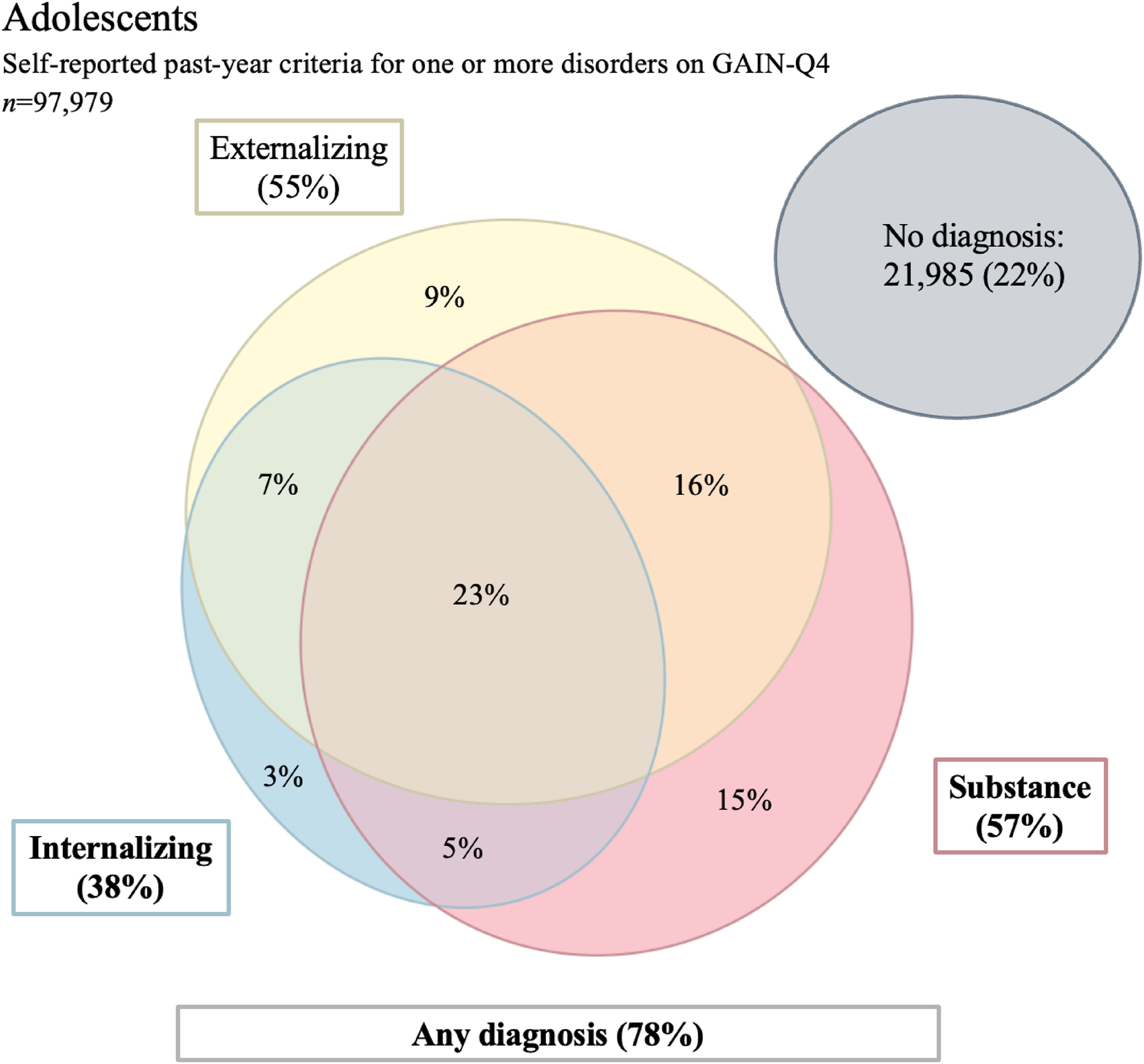
In addition to identifying general substance use and specific SUDs in adolescents, the GAIN‐Q4 can simultaneously recognize other psychiatric diagnoses. Co‐occurring MH disorders and SUDs are common in the adolescent population (
1,
30). In this sample, nearly half of the adolescents had an IMH or an EMH in addition to a SUD diagnosis (44%) and almost a quarter had co‐occurring SUD, EMH and IMH diagnoses (23%). The utility of the GAIN‐Q4 in adolescents is displayed by its ability to recognize problems associated with multiple psychiatric diagnoses (SE = 97%) in a short period of time unlike many diagnostic tools (
15,
31,
32). Identification and treatment of co‐occurring diagnoses is imperative, as the presence of multiple psychiatric disorders intensifies disease sequelae including academic problems, HIV risk behaviors, and suicide attempts (
33,
34). Outcomes for adolescents with co‐occurring disorders are poor despite receiving treatment at higher rates than those with an individual diagnosis (
30). Appropriate recognition and treatment planning tailored to an individual's diagnoses is critical in a population with high prevalence of co‐occurring disorders. The GAIN‐Q4 performed particularly well at identifying possible psychosis (SE = 100%, SP = 100%) and suicide attempts (SE = 95%, SP = 100%) both of which are important risk factors for future suicidal behavior (
35).
This study had numerous strengths, including a large sample, offering strong statistical power (
16). Additionally, the GAIN‐Q4 is based on a reliable, full‐length diagnostic tool (GAIN‐I) (
16), and provides users with treatment recommendations rooted in evidence‐based ASAM criteria in a shorter format than the GAIN‐I. Clinicians often struggle with management of care for adolescents dealing with substance use (
36). The GAIN‐Q4 alleviates this issue by offering a bridge from diagnosis directly to treatment planning. One limitation of this study was the use of self‐reported data. Results have the potential to be inflated due to the use of a subset of items in the GAIN‐Q4 scale to predict all the items typically reflected in the GAIN‐I (
16), with the goal of increasing efficiency. Lastly, the generalizability of results is reduced by a few factors. The sample used was diverse, but predominantly white (48%) and male (72%). Moreover, most participants were enrolled in specialized SUD, justice‐involved treatment, and MH care which potentially diminishes the applicability of these findings in other settings (
16). Future studies could include validating the reliability of the GAIN‐Q4 in other clinical environments, such as primary care offices, in order to strengthen the generalizability of the instrument. Future work should determine the effectiveness of these suggestions at initiating treatment for SUDs among adolescents. It should also be noted that the development and validation of the GAIN Q4 is just one component of a multiyear process being conducted in conjunction with the Connecticut DCF. Starting in 2024 the department and its network of providers is implementing the recommendations made by the GAIN‐Q4 in order to evaluate its impact on actual treatment and patient outcomes.
The results of this study support the utility of the GAIN‐Q4 in diagnosing SUDs in adolescents given the strong sensitivity (89%), specificity (93%), Cohen's kappa (0.80), and percent agreement (90%) for any SUD diagnosis. The GAIN‐Q4 also has the ability to identify the co‐occurring MH and SUD diagnoses common in the adolescent population (
1,
30). The reliability of the GAIN‐Q4 as a diagnostic measure should allow providers to feel comfortable using this abbreviated instrument to provide rapid and accurate diagnosis of SUDs in the adolescent population.