Developing an International Standard Set of Patient-Reported Outcome Measures for Psychotic Disorders
Abstract
Objective:
Methods:
Results:
Conclusions:
HIGHLIGHTS
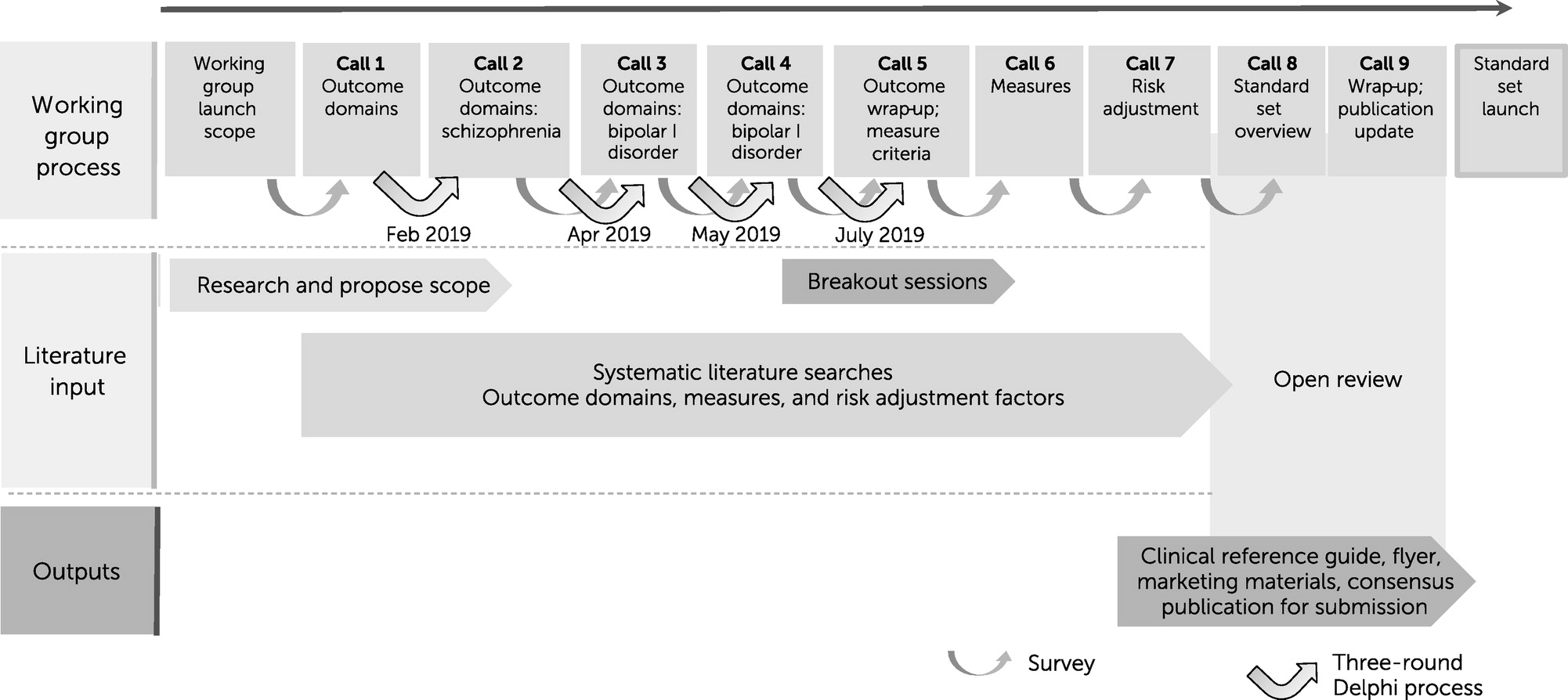
Methods
Service User Focus Groups
Systematic Literature Review to Identify Outcomes
Consensus Process
Identification of Potential Outcome Measures From the Systematic Literature Review
Assessment of PROMs
Breakout Sessions to Narrow the List of Measures
Risk Adjustment Factors
Open Review and Patient Validation
Results
Scope
Service User Focus Groups
Identifying PROs
Domain, Outcome, and Measure Selection by Consensus Working Group
| Domain and outcome | Definition | Outcome measurea | Administration timing | Data source |
|---|---|---|---|---|
| Symptoms | ||||
| Depressive symptoms | Mood or emotional state that is marked by feelings such as depressed mood, hopelessness, worthlessness, or guilt and a reduced ability to enjoy life | PHQ-9 (41) | Baseline and every 6 months | Patient |
| Suicidal ideation and behavior | Suicidal ideation, suicidal thoughts or behaviors, suicide attempts, most often accompanied by intense feelings of hopelessness or depression or by self-destructive behaviors | PHQ-9 (41) | Baseline and every 6 months | Patient |
| Positive symptoms | Change in thoughts or perceptions, including hallucinations, delusions, or disorganized thought | MCSI (40) | Baseline and every 6 months | Patient |
| Negative symptomsb | A withdrawal or lack of function not expected in a healthy person, including blunting of affect, poverty of speech and thought, apathy, anhedonia, reduced social drive, loss of motivation, lack of social interest, and inattention to social or cognitive input | ReQoL-20 (39) | Baseline and every 6 months | Patient |
| Mania, hypomaniac | Abnormally elevated mood state characterized by symptoms such as inappropriate elation, increased irritability, severe insomnia, grandiose notions, increased speed or volume of speech, disconnected and racing thoughts, increased energy and activity level, and inappropriate social behavior | ASRM (42) | Baseline and every 6 months | Patient |
| Sleep quality | Sleep problems resulting in decreased quality, including difficulty falling asleep, difficulty staying asleep, early morning awakening, and not feeling rested on waking up | PROMIS-Sleep (43) | Baseline and every 6 months | Patient |
| Relapse rate | Reemergence of symptoms or disorder after partial or complete recovery | Hospitalizations | Baseline and every 6 months | Clinician or patient |
| Recovery | ||||
| Personal recovery | A very personal process of changing one’s attitudes, values, feelings, goals, skills, or roles. A way of living a satisfying, hopeful, and contributing life, according to the CHIME domains: connectedness, hope and optimism, identity, meaning and purpose, and empowerment | ReQoL-20 (39) | Baseline and every 6 months | Patient |
| Quality of life | Individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, including independence, experiencing a fuller range of emotions, and life satisfaction | ReQoL-20 (39) | Baseline and every 6 months | Patient |
| Functioning | ||||
| Global functioning | Individuals’ social, occupational, and psychological functioning | WHODAS 2.0 (adult) (44); KIDSCREEN-10 (adolescent) (45) | Baseline and every 6 months | Patient |
| Social functioning | Individuals’ interactions with their environment, the quality of those interactions, and their ability to fulfill their role within such environments as work, social activities, and relationships with partners, families, or friends | WHODAS 2.0 (adult) (44); KIDSCREEN-10 (adolescent) (45) | Baseline and every 6 months | Patient |
| Role functioning | Ability to perform occupational activities or performance of functional tasks that support participation in the academic aspects of school | WHODAS 2.0 (adult) (44); KIDSCREEN-10 (adolescent) (45) | Baseline and every 6 months | Patient |
| Physical health | Measure of general medical health and well-being and overall satisfaction with general medical health | PHQ-15 (46) | Baseline and every 6 months | Patient |
| Treatment side effects | Effects of the prescribed medication, whether therapeutic or adverse, besides the intended treatment effect | GASS (47) | Baseline and every 6 months | Patient |
| Measure, abbreviation, and relevant study | No of items | Validitya | Reliabilitya | Sensitivity to changeb |
|---|---|---|---|---|
| 9-item Patient Health Questionnaire (PHQ-9) (41, 48, 49) | 9 | ++ | + | ** |
| Modified Colorado Symptom Index (MCSI) (40) | 14 | + | ++ | × |
| 20-item version of Recovering Quality of Life (ReQoL-20) (39, 50) | 20 | NAc | ++ | * |
| Altman Self-Rating Mania Scale (ASRM) (42) | 5 | NA | ++ | × |
| PROMIS Short Form V1.0 Sleep Disturbance 4a (PROMIS-Sleep) (43) | 4 | ++ | + | × |
| 12-item version of the WHO Disability Assessment Schedule 2.0 (WHODAS 2.0 [adult]) (44) | 12 | + | + | × |
| 10-item KIDSCREEN (adolescent) (45) | 10 | ++ | + | × |
| 15-item Patient Health Questionnaire (PHQ-15) (46, 49) | 15 | + | ++ | * |
| Glasgow Antipsychotic Side-Effect Scale (GASS) (47) | 22 | ++ | ++ | × |
Evaluation of Measures
Time Point Recommendations
Risk Adjustment Factors
| Risk adjustment area, patient population, and measure | Supporting informationa | Administration timing | Data source |
|---|---|---|---|
| Demographic factor | |||
| All patients | |||
| Year of birth | NA | Baseline | Patient reported |
| Sex | Sex at birth | Baseline | Patient reported |
| Gender identity | NA | Baseline | Patient reported |
| Sexual orientation | NA | Baseline | Patient reported |
| Socioeconomic status | Adults, highest level of education completed; adolescents, highest level of education completed by parents (proxy to be used) | Baseline, transition to adult services, and annually if still in education | Patient reported |
| Work or education status | NA | Baseline and annually | Patient reported |
| Housing status | NA | Baseline and annually | Patient reported |
| Living arrangement | NA | Baseline and annually | Patient reported |
| Ethnic minority group or marginalization | NA | Baseline | Patient reported |
| Adult patients and adolescent patients where appropriate | |||
| Contact with law enforcement | To be administered to adolescents only when appropriate to do so and for whom this measure would not cause unnecessary distress. Baseline, ever been convicted (lifetime); annually, ever been convicted (in past 12 months) | Baseline and annually | Patient reported |
| Clinical factor | |||
| All patients | |||
| Comorbid conditions | Based on the Self-Administered Comorbidity Questionnaire (54) | Baseline and annually | Patient reported |
| Hospitalizations | Number of lifetime hospitalizations related to the target condition | Baseline | Administrative data |
| Adult patients and adolescent patients where appropriate | |||
| Adverse life experiences | To be administered to adolescents only when appropriate to do so and for whom this measure would not cause unnecessary distress | Baseline and transition to adult services | Patient reported |
| Intervention factor | |||
| All patients | |||
| Intervention setting | NA | Baseline and annually | Clinical |
| Intervention type | NA | Baseline and annually | Clinical |
Open Review and Patient Validation
Discussion
Conclusions
Supplementary Material
- View/Download
- 343.13 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

