Identifying individuals at risk for problematic opioid use is often difficult in clinical practice. Patients may have multiple providers for several conditions that each increase their risk for such use, and the providers deciding whether to prescribe opioids may lack the time to conduct comprehensive medication reconciliations or risk assessments. Furthermore, screening tools, although useful, can be time consuming to use and may not be practical in all settings or for all patients. Recent research has suggested that analytics using electronic medical records may identify patients at an increased risk for overdose or suicide-related events (
1). Identifying the population at risk for developing opioid dependence by using already available information would allow time and resources to be devoted to the individuals who need them most (
2). Previous research has also used data from electronic health records to predict problematic opioid use, with encouraging results (
3–
5).
One approach to addressing the risk of problematic opioid use is a direct-to-consumer mailing intervention, which provides information to the patient and facilitates collaboration with the individual’s provider, prescriber, or physician to best manage their health. This approach has been shown to be effective, possibly by empowering individuals to initiate conversations with their physician (
6,
7). For example, one direct-to-consumer education initiative among older adults increased discussions and shared decision making with their prescribers and significantly reduced benzodiazepine prescriptions (
8).
A variation of this strategy uses a mailing intervention sent to prescribers whose prescribing practice differs substantially from that of their peers. Although some research has found limited effectiveness of the peer comparison approach (
9), observations from other studies suggest that these types of interventions result in significant and lasting changes in prescribing patterns (
10). A recent randomized controlled trial reported that clinicians who received a letter informing them of a patient’s death and including information on safe prescribing practices reduced high-intensity prescribing and prescribed fewer new opioids (
11).
The purpose of this study was to pilot a newly developed Opioid Risk Stratification Tool to identify individuals who are likely at moderate to high risk for unhealthy opioid use and to examine the impact of a mailing and engagement intervention with the goal of reducing opioid medication use and associated mortality rates.
Methods
Risk Stratification Tool
The proprietary Relias Opioid Risk Stratification Tool stratifies individuals into low, medium, or high risk for problematic opioid use. The tool uses an algorithm, developed with logistic regression, that contains 96 data points, such as chronicity of use, multiple opioid prescriptions, use of multiple pharmacies, concurrent opioid use with a diagnosis of opioid use disorder or other substance use disorder, and use of opioids at a high dose without a supporting diagnosis. “Problematic opioid use” is identified when any one of the following occurs in the outcome period: the opioid misuse indicator—signaling aberrant behaviors around drug acquisition and abuse at the end of the outcomes period (e.g., multiple prescribers or pharmacies, malingering or somatization, or chronic or high opioid use without supporting diagnosis)—is triggered; a specific opioid use disorder is diagnosed; some other pattern of misuse emerges that is not a quality indicator (e.g., multiple early refills or for small amounts); or an assessment indicates opioid misuse, addiction, or use disorder. The first three items are extracted from claims data (e.g., pharmacy claims and diagnosis codes), and the fourth is extracted from scores from the Daily Living Assessment–20 for alcohol and drug use (
12).
The tiers of problematic use were calculated by using raw risk scores on the basis of a classification model. The algorithm includes 96 variables that contribute to the raw risk score. The resulting score is a value ranging from 0.000 to 1.000, with higher scores indicating high probability of opioid misuse in the 3-month follow-up period. Finally, to determine the risk tiers, the raw risk score is converted to a percentile ranking on the basis of the report period and population. This tool helps inform decisions at the population level and helps focus interventions to the patient or prescriber level.
To validate the risk stratification model, a receiver operating characteristic curve was used to assess the model’s performance in terms of sensitivity and specificity. The model was built with one data set (N=1.4 million monthly patient records) and was validated with another (N=453,000 monthly patient records), such that none of the same patients were in both the training and validation samples. Both samples comprised records from behavioral health organizations in the Midwest or Eastern U.S. regions that serve Medicaid-insured adults and children—similar to the organizations of the intervention and control groups in this study. For the final predictive risk model on the patient set used for validation, the area under the curve was 0.89, suggesting robust performance of the model.
Study and Control Groups
For this study, we included all members of a local managed care organization (MCO) in the Mid-Atlantic region of the United States who were at moderate to high risk for opioid dependence, according to the Opioid Risk Stratification Tool, in January through March 2019. The control group was a convenience sample derived from a different MCO in the same region and included members who were at moderate to high risk for opioid dependence in January through March 2019. All participants in both groups were eligible for Medicaid during the entire study period. The Center for Outcomes Analysis Institutional Review Board approved this study, which involved deidentified data.
Intervention
Mailing.
A mailing intervention was sent to members at risk for opioid dependence in the intervention group and their prescribers in July 2019; the mailing was intended to be an informative, rather than punitive, measure. Members were sent a letter suggesting that they meet with their prescriber and providing talking points for the visit. Prescribers were sent a letter indicating that they had a patient at moderate to high risk for problematic opioid use and requesting that they meet with the patient. A copy of the Screener and Opioid Assessment for Patients With Pain (
13,
14), a short in-person screening tool, was also provided, along with an overview of where to refer patients for treatment. Also included was a postcard (
15) to be returned with yes-or-no responses to the following questions: Did you screen the member for substance use disorder and opioid use disorder? Did the score indicate the need for behavioral health treatment? Was a behavioral health treatment referral made?
Engagement.
A care coordinator engaged members in the intervention group who were still at risk for opioid dependence in September 2019 by telephone and asked them several questions, including whether they had talked with their physician about the potential adverse effects and risks of opioids. The care coordinator also provided tips to reduce the risk for opioid addiction. (See the online supplement to this article for sample intervention materials.)
Data and Outcome Measures
Data for analysis were collected from Medicaid claims from Mid-Atlantic providers between November 2018 and May 2019 for the preintervention period and between November 2019 and May 2020 for the postintervention period. The same period was compared across years to avoid any potential seasonality effects in health care use. In addition, we also present results for claims where the postintervention period was truncated to end in March 2020 to account for the onset of the COVID-19 pandemic.
Opioid use.
To standardize opioid use across the population, we calculated morphine milligram equivalents (MMEs) and determined the percentage of patients who showed a reduction in MMEs (defined by comparing total MMEs in each period) or MME discontinuation between baseline and postintervention. The number of members with multiple opioid prescriptions (defined as two or more active opioid prescriptions with a minimum of a 60-day overlap period), multiple prescribers for opioids (defined as four or more prescribers), and concurrent opioid and benzodiazepine prescriptions (minimum of a 60-day overlap period) were also examined.
Mortality rate.
Mortality rate was derived from the number of opioid-related overdoses and deaths, defined with ICD-10 poisoning codes, in the postintervention period reported in the claims data.
Statistical Analysis
We compared the change in several measures of opioid use between the intervention and control groups during the preintervention period before letters were sent to any prescribers and patients and during the postintervention period.
To analyze receipt of multiple opioid prescriptions, having multiple prescribers of opioid prescriptions, and taking both opioids and benzodiazepines, we used logistic regression, conducted within the generalized estimating equations statistical framework to account for multiple observations (GENMOD procedure in SAS, version 9.4), in order to estimate change in the odds of the outcome at postintervention relative to preintervention. For prescription of any opioids, we used a conventional multivariate binary logistic regression (LOGISTIC procedure). Because taking any opioids was an inclusion criterion, all participants were taking opioids preintervention, eliminating the need for repeated-measures analysis. We used the same logistic regression for MME reduction, where the variable reflected MME reduction from pre- to postintervention versus no reduction. Age and comorbid general medical conditions were controlled for in all analyses because descriptive analyses revealed significant group differences in these two variables. The results were interpreted as ORs, with an OR <1.00 indicating reduced risk and an OR >1.00 indicating increased risk. ORs were considered statistically significant at a two-tailed p<0.05, when the lower and upper limits of the 95% CI did not span 1.00. No data were missing.
Results
In total, 318 participants were eligible. The Opioid Risk Stratification Tool identified 131 managed care members in the intervention group and 187 members in the control group as being at moderate to high risk for opioid dependence. In total, 167 prescribers were associated with the members in the intervention group and received the mailing intervention. Only one prescriber returned the postcard, so it was not possible to determine how many prescribers met with patients to assess whether further screening was conducted. Fifty-four members were still in the moderate-to-high-risk category after the mailing intervention, and all were subsequently contacted by a care coordinator for the engagement portion of the intervention. The sample obtained after truncating the postintervention period to account for the onset of the COVID-19 pandemic included 130 participants in the intervention group and 187 in the control group.
Characteristics of the participating members are presented in
Table 1. Individuals in the control group were on average significantly older than those in the intervention group and had a higher number of comorbid general medical conditions. The two groups did not differ significantly in terms of sex or comorbid behavioral health conditions. MME reduction from pre- to postintervention was similar for the two groups. The proportion of those with multiple opioid prescriptions was greater in the control compared with the intervention group, whereas the proportion of those with a concurrent prescription of opioids and benzodiazepines was higher in the intervention group. The characteristics of the sample with a truncated postintervention time were essentially identical to those of the main sample.
Opioid Use
As determined by the inclusion criteria, all participants were receiving opioids at preintervention. At postintervention, the odds of having any opioid prescription were significantly lower in the intervention group compared with the control group (OR=0.55, 95% CI=0.39–0.78, p<0.001). Sixty participants (46%) in the intervention group and 120 participants (64%) in the control group were still receiving opioids postintervention. For the sample with a truncated postintervention period, the odds of receiving any opioid prescription at postintervention were lower for the intervention group compared with the control group (OR=0.50, 95% CI=0.35–0.70, p<0.001); 57 participants (44%) in the intervention group (data for one participant were not available in the truncated period) and 120 (64%) in the control group were still receiving any opioids postintervention. No significant differences were detected in the reduction in MME from pre- to postintervention by group (OR=0.99, 95% CI=0.69–1.42).
At baseline, 165 (88%) of those in the control group and 103 (79%) of those in the intervention group were receiving multiple opioid prescriptions, 77 (41%) versus 42 (32%) were receiving opioids from multiple prescribers, and 24 (13%) versus 33 (25%) were receiving concurrent opioid and benzodiazepine prescriptions. The difference between the two groups in multiple prescriptions of opioids was statistically significant at baseline (p=0.021) and postintervention (p=0.003).
Results for changes in the odds of these outcomes are presented in
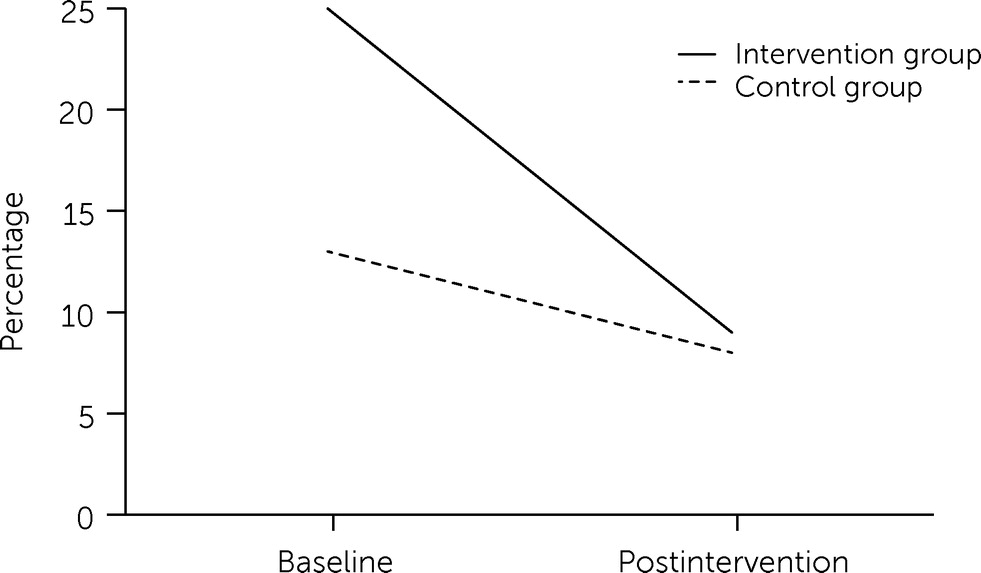
Table 2, revealing significant reductions in the odds of having multiple prescriptions (OR=0.17, p<0.001) and multiple prescribers (OR=0.31, p<0.001) between the pre- and postintervention time points overall, but these reductions did not differ statistically significantly between the two groups. The intervention group had significantly higher odds of concurrent prescription of opioids and benzodiazepines (OR=2.75, p=0.001); we observed a significant reduction in the odds of having concurrent opioid and benzodiazepine prescriptions between pre- and postintervention for both groups (OR=0.58, p=0.019), and this reduction was greater in the intervention compared with the control group (OR=0.49, p=0.042;
Figure 1). The results for the sample with a truncated postintervention period (
Table 2) were essentially identical to those of the main sample, but the reduction in the concurrent prescription of opioids and benzodiazepines by group was no longer statistically significant.
Mortality Rate
No opioid-related overdoses or deaths were reported in the claims data for either group.
Discussion
In this study, we evaluated the impact of a mailing and engagement intervention on opioid medication use, risky opioid medication practices, and mortality rate in a population identified via predictive analytics as being at increased risk for developing opioid use disorder. Two key findings were that the intervention resulted in a reduction in prescription of concurrent opioids and benzodiazepines and a greater likelihood of discontinuing opioid medication. Other well-known substandard prescribing practices were not significantly influenced by the intervention.
That the intervention was significantly associated with a decrease in the number of individuals with both opioid and benzodiazepine prescriptions supports previous literature showing that direct-to-consumer interventions can have beneficial impacts on reducing benzodiazepine prescribing (
6–
8). A longer study period or more detail in the letters about why the patient was at risk may be needed to effect changes in other prescribing practices, such as having multiple opioid prescribers or multiple opioid prescriptions. Both groups had significant decreases in prescribing practices over time, which may be attributable to systemwide initiatives such as the prescription drug monitoring programs in place in the region. Our results also showed that the intervention group had a higher proportion of individuals who discontinued opioids compared with the control group. This finding suggests that alerting members and their prescribers of an increased risk for problematic opioid use may lead to shared decision making between patients and providers and a reevaluation of treatment.
The intervention in this study used a proactive approach to reach individuals potentially at risk for opioid dependence. This method differed from traditional approaches that tend to identify individuals who have developed sequelae (
16). Fraser and Plescia (
17) highlight the importance of using a population health perspective to target individuals at high risk, representing a primary prevention–focused approach to addiction. Other recent studies have begun to use algorithms to identify individuals who may be at increased risk for problematic opioid use (
3–
5), but none of these studies has used these tools to identify a group to receive an intervention to reduce risk.
Patient agency—involving individuals in their own care and encouraging them to engage with their physician to find the optimal treatment strategy while minimizing risks—is another important component of successful prevention efforts. Despite the widespread opioid epidemic, many individuals report poor communication around opioid risks and pain management (
18), and many individuals either are unaware of the risks of opioids (
19) or underestimate their risk for developing opioid use disorders (
20). This intervention was intended to educate both members and prescribers, without being punitive or demeaning, in order to encourage informed shared decision making.
This intervention appears to be most successful in influencing the aspects of opioid use that are directly within the provider’s control—prescription of opioids and concomitant prescription of opioids with benzodiazepines. Multifaceted interventions are necessary because the risks for hazardous opioid use are themselves multifaceted. Education can change the behavior of prescribers (
21); however, also engaging patients creates another level of outreach. This study included both low- and high-touch interventions. Individuals are unique, and although some may be prompted to change because of an educational letter, others may need the motivation provided by contact with a care coordinator. These differing interventions may also save costs. Our intervention started with a lower-cost intervention (mailing only), which reduced risk for some people (N=77), and only 41% (N=54) of members needed the more expensive high-touch intervention (i.e., mailing plus care coordinator engagement). A tiered intervention likely uses limited resources efficiently.
Limitations
These results should be interpreted in the context of several methodological limitations. First, participants were not randomly assigned to the intervention or control conditions. Therefore, we could not account for possible group differences that affected outcomes. However, the two organizations comprising the intervention and control groups were similar in size and covered similar populations in the same geographic location. Second, this study was based on claims data, which may contain inaccurate coding and incomplete claims or may not capture deaths. Third, the study contained a relatively short 6-month follow-up period; a longer study period may yield greater differences between groups by allowing for further advancement through the stages of change. Fourth, overall health care resource utilization, including use of providers, emergency department use, and hospital visits, was not evaluated for changes or between-group differences. Fifth, although feedback was requested from providers in the form of an anonymous prestamped postcard, this approach was not successful, limiting our ability to understand provider engagement and to ascertain whether the in-person assessment was conducted.
Future Research
This study sets the stage for further investigation. Increasing the follow-up time to 12 months would allow for a more extended period of change. The impact of offering patients an anonymous contact line to confidentially ask questions to a provider could be a valuable addition for individuals who may be reluctant to address difficult issues face to face. Finally, other interventions that may affect opioid prescribing practices should be studied.