The national crises of depression, suicide, and opioid-related harms such as overdose are closely linked (
1). Pain and depression are among the most common medical conditions (
2,
3) and are frequently comorbid. In primary care settings, 27% of patients with pain have concurrent major depression, and its prevalence increases to 52% among patients in specialty pain care settings (
4). Pain and depression are also independent risk factors for self-harm and suicide. Individuals with chronic pain are two to three times more likely to report suicidal behaviors or to die by suicide (
5), and chronic pain is present in 9% of suicide decedents (
6). Among adults with chronic pain, comorbid depression elevates suicide risk fourfold (
7).
Opioid treatment for pain further complicates the interactions among pain, depression, and self-harm. Certain opioid use patterns, specifically, high opioid doses, prescription opioid misuse, and opioid use disorder (
1), are associated with increased risk for suicidal thoughts, behaviors, and death (
8,
9). Comorbid depression among individuals with pain who receive opioids may further amplify these risks. Individuals with depression taking chronic opioids are two to three times more likely to misuse opioid medications than are individuals without depression (
10), and such misuse increases suicidal behavior risk (
9,
11,
12). The risk for other opioid-related harms, such as nonfatal and fatal overdoses, is also higher among individuals with depression (
13,
14). Evidence suggests that opioids figure prominently in suicide dynamics: one-third of suicides by intentional overdose in 2017 involved opioids (
15); moreover, as many as 20%–30% of nonfatal and fatal opioid overdoses may represent suicide attempts and death, respectively (
16,
17).
Although comorbid depression substantially increases overdose and suicide risk, it remains underdiagnosed and undertreated among individuals with chronic pain (
18,
19). Increasing access to depression treatment for individuals receiving opioids has therefore emerged as one potentially promising approach to preventing overdoses and suicide.
In the general population, physicians’ recognition and treatment of depression can lower suicide rates (
20); in addition, there is a lower risk for suicidal behavior among individuals with opioid use disorder receiving mental health treatment compared with those receiving no such treatment (
1). However, there is scant evidence on the extent to which depression treatment can reduce the incidence of suicide, overdose, and other opioid-related harms among patients who receive opioids for pain but who do not necessarily have opioid use disorder. Clinical trials have not reported how antidepressant use affects this subpopulation, and using observational data presents challenges for examining the relationship between antidepressant use and adverse events. The first challenge is selection into treatment. Individuals receiving antidepressants are likely to have more severe underlying depression symptoms than individuals not receiving antidepressants (
21); therefore, they are at higher risk for adverse health outcomes independently of receiving treatment (
22). Accurately measuring and adjusting for depression severity is also difficult in observational studies using administrative data (
23).
In theory, quasi-experimental approaches, in which antidepressant prescription use is quasi-randomly assigned independently of depression severity or receipt of opioids, offer a potential solution. However, in practice, pain treatment and behavioral health treatment are closely linked: the majority of both mental health and chronic pain treatment is delivered in primary care settings (and therefore likely by the same provider) (
24–
26), and antidepressants may be prescribed for chronic pain with or without comorbid depression (
27,
28). It is therefore difficult to identify sources of random variation in antidepressant use that do not simultaneously affect opioid treatment and other aspects of behavioral health care.
A second challenge is that the risks for overdose and suicide after treatment with opioids vary substantially over time (
29). Antidepressant medication efficacy also varies over time. Antidepressants may somewhat improve depression symptoms within the first 1–2 weeks of treatment, but a clinically meaningful treatment response or remission of symptoms typically occurs only after 4–6 weeks (
30–
37). Therefore, to assess the risk for adverse health outcomes, knowing whether receipt of opioids and receipt of antidepressants overlap and how early or late the overlap occurs during treatment is essential.
In our study, we used a national commercial claims database to examine the risk for overdose and self-harm among individuals with a history of depression treatment who received opioid analgesics between 2007 and 2017. We compared risks for adverse outcomes between those who filled antidepressant prescriptions and those who did not. We leveraged the fact that efficacy of antidepressant treatment varies over time, with clinically meaningful treatment responses not commonly occurring immediately after treatment is initiated, to separate the risk for adverse events due to selection into treatment from the causal impact on adverse events due to antidepressant treatment itself. To our knowledge, no previous study has examined whether antidepressant treatment can mitigate the risk for overdose and self-harm among individuals receiving prescription opioids for pain.
Methods
Data Source and Analytic Sample
We used medical and pharmacy claims from January 2007 through December 2017 recorded in the Optum Clinformatics Data Mart database, a comprehensive, deidentified claims data warehouse that includes information on individuals in employer-sponsored plans, Affordable Care Act Exchange plans, and Medicare Advantage plans in all 50 U.S. states and Puerto Rico. The database represents 39.0 million unique patients, including 16.7 million patients who filled at least one opioid analgesic prescription between 2007 and 2017. To mitigate selection bias due to unobserved depression severity, we restricted our analysis to adults ages ≥18 years with an emergency department (ED) visit or inpatient stay whose claim contained a diagnosis of depression before their first filled opioid prescription. We excluded individuals diagnosed as having bipolar disorder or a psychotic disorder in the 6 months before or the 12 months after their first filled opioid prescription; we also excluded individuals continuously enrolled for <6 months before their first filled opioid prescription. (A table showing inclusion and exclusion diagnoses is available in an
online supplement to this article.)
Our analytic sample comprised opioid analgesic episodes initiated between February 2007 and December 2017. An episode was defined as starting with the first filled opioid prescription after a preceding period of at least 30 days during which the patient was not in possession of an opioid from a prescription fill (e.g., if a patient had previously filled an opioid prescription, the days’ supply from that prescription had been exhausted before that period) and as ending 30 days after the days’ supply of any previous opioid prescription was exhausted and no new opioid prescription was filled. Individuals could contribute multiple opioid episodes. The institutional review board of Indiana University determined this study to be exempt from review.
Measures
The outcome of interest was the time from the beginning of an opioid episode until an adverse event, defined by
ICD-9 and
ICD-10 codes for opioid overdose (identified in claims as “opioid poisonings”), overdoses of nonopioid controlled substances (e.g., benzodiazepines or nonopioid illicit drugs), overdoses of noncontrolled or unspecified substances, and self-harm unrelated to overdose (see the
online supplement). Adverse events could occur during or after an opioid episode. Regardless of the opioid episode’s length, we followed up individuals from the opioid episode onset until an adverse event, loss to follow-up, or week 52 (the time horizon), whichever came first. Those who were lost to follow-up or reached week 52 were censored at that time point. The key independent variable, which defined the exposure of interest, was filling an antidepressant prescription. Other independent variables included patient sex and age.
Statistical Analysis
We estimated standard, discrete-time, proportional hazards survival models to characterize hazard and survivor functions that quantified the association between antidepressant fills and adverse events after the start of a new opioid episode. We estimated the hazard function (i.e., the probability that an adverse event would occur during a week, our unit of time) as a multivariable logistic regression model in which the person-week was the unit of analysis. We specified the model with a set of indicators for the number of weeks since the opioid episode began, annual calendar year indicators, and time-invariant individual characteristics, measured at the start of the opioid episode (i.e., patient sex, age, and the length of a patient’s continuous insurance enrollment at the beginning of the episode).
We included two time-varying weekly antidepressant measures: an indicator that the patient had filled an antidepressant prescription and had remaining days’ supply and an indicator that the patient had remaining days’ supply of antidepressants and had been receiving antidepressants for at least 6 weeks.
Use of these two antidepressant measures enabled us to differentiate between a period early in antidepressant treatment, when individuals were unlikely to be receiving full clinical benefits, and a period 6 weeks after individuals began treatment, when they were likely to be receiving the medication’s full benefits. This differentiation allowed us to distinguish analytically between adverse event selection (the extent to which those with more severe depression are both more likely to receive antidepressants and more likely to be at increased risk for an adverse event) and antidepressant efficacy (the reduction in risk after the medication becomes efficacious). Because antidepressant prescriptions can be filled any time before or after opioid treatment commences, we identified selection and efficacy effects throughout the opioid episode. To account for intra-episode and intra-person correlation across observations, we calculated Huber-White robust standard errors clustered at the person level.
We first estimated versions of the model that included any adverse events as the outcome. Next, we estimated separate models for each type of adverse event defined earlier by using a competing risks approach in which we censored all observations at the time of the first adverse event if it was not the event of interest (
38).
Results
Sample Characteristics
We identified 283,374 adults with 336,599 opioid treatment episodes who had a previous ED visit or inpatient stay with a diagnosis of depression. Approximately half (N=144,052, 50.8%) filled an antidepressant prescription at least once in the 12 months after their opioid treatment episode began.
Table 1 shows the characteristics of individuals who received an antidepressant and those who did not. Individuals receiving an antidepressant were more likely to be female and age ≥45 years.
Of the 283,374 adults, 8,203 (2.9%) experienced a total of 47,486 adverse events. These events were overdoses with noncontrolled or unspecified substances (N=33,596, 70.7%), overdoses with nonopioid controlled substances (N=5,796, 12.2%), opioid overdoses (N=4,748, 10.0%), and self-harm events unrelated to overdoses (N=3,346, 7.0%). The most frequent adverse event recorded was “poisoning by unspecified drug or medicinal substance” (
ICD-9 code 977.9) (see the
online supplement). Individuals with an opioid episode who received an antidepressant were more likely to experience an adverse event than individuals not receiving an antidepressant (3.6% vs. 2.2%) (
Table 1); this pattern was observed for each type of adverse event and likely reflected selection due to antidepressants being prescribed for those with more severe depression.
Survival Analysis
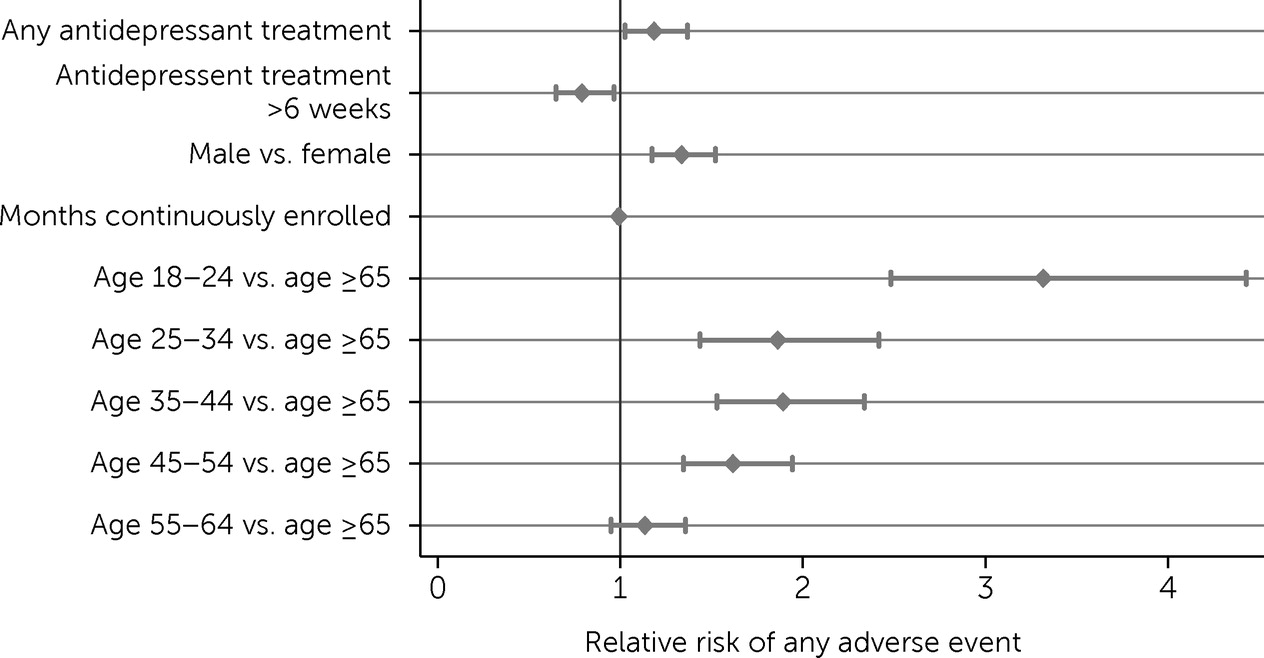
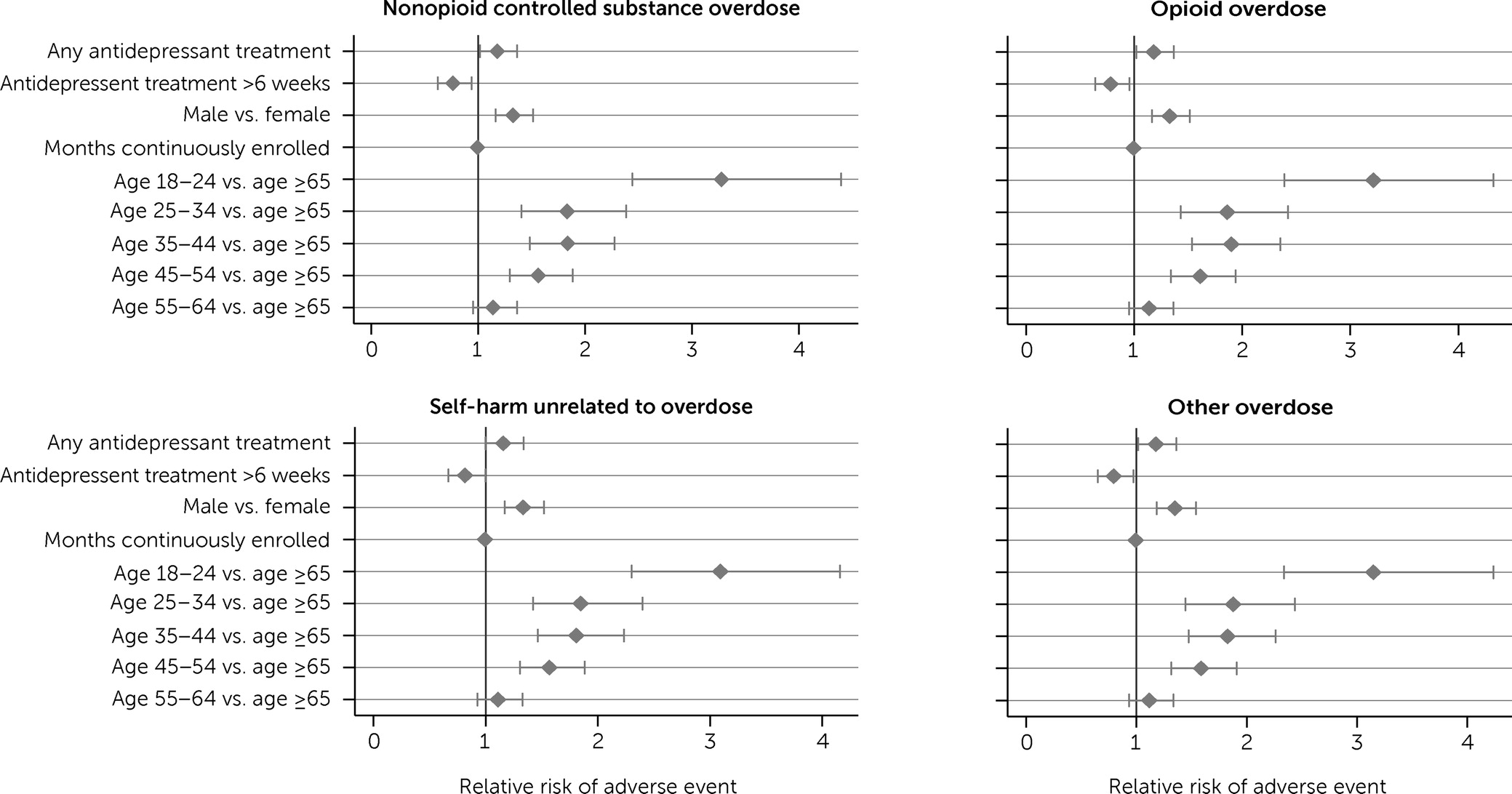
In our discrete-time survival models, all categories of adverse events were more likely among men than women, and the risk for adverse events was highest among individuals ages 18–24.
Figures 1 and
2, which show adjusted odds ratios (AORs) for predictors from our survival models for any adverse event and specific types of adverse events, respectively, illustrate that risk decreased with increasing age.
After we adjusted analyses for age and sex, individuals receiving antidepressants had a greater risk for any adverse event within the first 6 weeks of treatment (AOR=1.19, 95% confidence interval [CI]=1.03–1.37) as well as for specific types of adverse events, including opioid overdoses (AOR=1.18, 95% CI=1.02–1.37), overdoses with nonopioid controlled substances (AOR=1.18, 95% CI=1.02–1.37), and overdoses with noncontrolled or unspecified substances (AOR=1.18, 95% CI=1.02–1.36); however, they did not experience a greater risk for other self-harm events (AOR=1.16, 95% CI=1.00–1.34) (
Table 2). These findings likely represent much of the higher risk for adverse outcomes associated with selection into treatment (e.g., a clinician choosing to prescribe an antidepressant because of symptom severity) because the adverse events occurred in the initial 6 weeks of antidepressant use, before many individuals were likely to receive the full clinical benefits of the medication.
However, antidepressant treatment after these first 6 weeks was associated with a reduced risk for adverse events overall (AOR=0.79, 95% CI=0.65–0.97) as well as reduced risks for opioid overdoses (AOR=0.78, 95% CI=0.64–0.96), overdoses with nonopioid controlled substances (AOR=0.76, 95% CI=0.62–0.94), overdoses with other substances (AOR=0.79, 95% CI=0.65–0.97), and other self-harm events (AOR=0.82, 95% CI=0.67–1.00). These findings further suggest that the beneficial impact of antidepressants is large enough to overcome the likely concerns about selection into treatment.
Discussion
Risks for overdose and self-harm are substantially higher among patients with comorbid pain and depression, particularly among those receiving opioids. Effective treatment of depression, such as antidepressants, may reduce opioid-related harms among individuals with a history of depression who are prescribed opioids. We found evidence consistent with these effects: individuals with a history of depression who were receiving opioid analgesics had a significantly lower risk for overdose and self-harm after they had been taking antidepressants for at least 6 weeks, the point at which they were likely experiencing the medication’s full benefits.
Measuring the impact of antidepressants in observational studies is challenging because of confounding by unobserved differences in depression severity. Specifically, individuals with more severe depression are more likely to be prescribed antidepressants than are others (
39), and individuals with more severe depression are more likely to have adverse events such as overdose and self-harm. As a result, individuals who receive antidepressants are more likely to have higher rates of adverse events, including a greater risk for overdose and self-harm, than those who do not receive antidepressants.
In our analysis, we used two strategies to mitigate this confounding. First, we restricted our sample to individuals with a history of a previous ED or hospital visit related to depression to limit our sample to a population with a history of more severe depression. Second, we capitalized on the clinical fact that antidepressants typically do not achieve full efficacy in many individuals until after the first 6 weeks of treatment. We examined differences in adverse events between those receiving antidepressants and those not receiving antidepressants, but we distinguished the first 6 weeks of antidepressant treatment from later periods. For the purposes of this analysis, we assumed that antidepressants had no immediate effect on adverse events, enabling our strategy to separate the effects of selection into treatment, which are present throughout an individual’s course of antidepressant treatment, from the benefits of antidepressants, which we assumed would be present after the first 6 weeks of treatment. Furthermore, because the timing of an antidepressant episode varies relative to opioid analgesic episodes, our approach identified the benefits of antidepressants with respect to the relative risk for adverse events throughout the opioid analgesic episode.
As expected, we found that individuals with depression who received antidepressants had a higher risk for adverse events before the antidepressants were fully effective: the odds of having an adverse event in the first 6 weeks after starting antidepressants were higher than for those not receiving antidepressants (AOR=1.19). We also found that the odds of having an adverse event were lower for those receiving antidepressants for ≥6 weeks compared with those receiving antidepressants for <6 weeks (AOR=0.79). We found comparable lower risks for opioid overdose, overdoses with nonopioid controlled substances, overdoses with other substances, and nonoverdose self-harm. These results suggest that antidepressant treatment may reduce the risk for overdose and self-harm among individuals with a history of depression who take opioids.
This study’s findings must be considered in the context of its limitations. First, although claims data have notable strengths relevant to an observational study of this nature, including large sample sizes for examining relatively infrequent events and detailed information on health care utilization, they provide only limited information about an individual’s clinical status, in particular disease severity. Therefore, we could not determine whether antidepressant and opioid treatments were clinically appropriate. We also could not determine at what point an individual received the full pharmacologic benefits of the antidepressant; we chose 6 weeks from the initial prescription fill, given a range of 4–8 weeks for the benefits of antidepressants to manifest that is commonly reported in the literature and the recognition that some individuals may not start taking antidepressants immediately after filling the prescription. To the extent that we have mischaracterized the lag between prescription fill and full pharmacologic benefit, our results were biased toward a null outcome. We attempted to mitigate bias due to unobserved depression severity by restricting our sample to individuals with a previous ED visit or hospitalization related to depression, suggesting moderate to severe disease.
Second, we observed only adverse events resulting in a medical encounter and hence a claim. We did not observe overdoses or self-harm events for which an individual received no medical care.
Third, measurement errors may have occurred. Specifically, we note that the single most common type of adverse event recorded in our data was overdose by unspecified substances (i.e., ICD-9 code 977.9 or ICD-10 code T50.901A). It is possible that this category included overdoses with controlled substances that have not been coded as such, perhaps because their presence had not been confirmed in a laboratory, because they were present in combination with other substances, or because the patient’s condition was simply misdiagnosed. A limitation of administrative data is the accuracy and specificity with which different types of adverse events are coded. In future research, electronic health record data or other data sources that contain laboratory values and progress notes might help to determine the specific types of overdoses or other adverse events that can be mitigated by antidepressant treatment among individuals taking opioids.
Despite these limitations, our study makes several contributions. To our knowledge, it is the first to examine how antidepressant treatment affects the likelihood of adverse health events among individuals prescribed opioid analgesics. In addition, we used a novel approach to adjust for selection into antidepressant treatment. We found that antidepressant treatment for at least 6 weeks may reduce the risk for overdoses and self-harm unrelated to overdoses in addition to improving depression symptoms and health-related functioning.
Conclusions
These findings have implications for pain treatment in clinical settings. Our study’s findings highlight the importance of universal screening for mood disorders among individuals receiving opioid analgesics, as is currently recommended by leading clinical practice guidelines (
40,
41), and the importance of promptly providing evidence-based depression treatment, when appropriate, with close patient follow-up. The efficacy and feasibility of this potentially promising harm-reduction approach warrant further investigation.
Acknowledgments
The authors thank Mary Vaiana, Ph.D., and Hilary Peterson, B.A., both of RAND Corporation, for their feedback and editorial assistance on earlier versions of the manuscript.