Articles identified included review articles, clinical guidelines, several primary publications, and abstracts of clinical trial data. A subsequent search of available databases and information confirmed long-acting risperidone as the only long-acting atypical agent. The terms used in the search criteria for this article may have limited the ability to find information about long-acting second-generation antipsychotic agents for indications other than schizophrenia. In addition to the data obtained from the literature search, a discussion by a panel of experts, which included the authors, was used to inform the suggestions presented in this article. The discussion took place at a meeting held in Dublin, Ireland, in May 2003 that was sponsored by an educational grant from Johnson & Johnson. Physicians are, of course, encouraged to use their own clinical judgment in conjunction with the recommendations presented here.
Efficacy and safety of long-acting risperidone
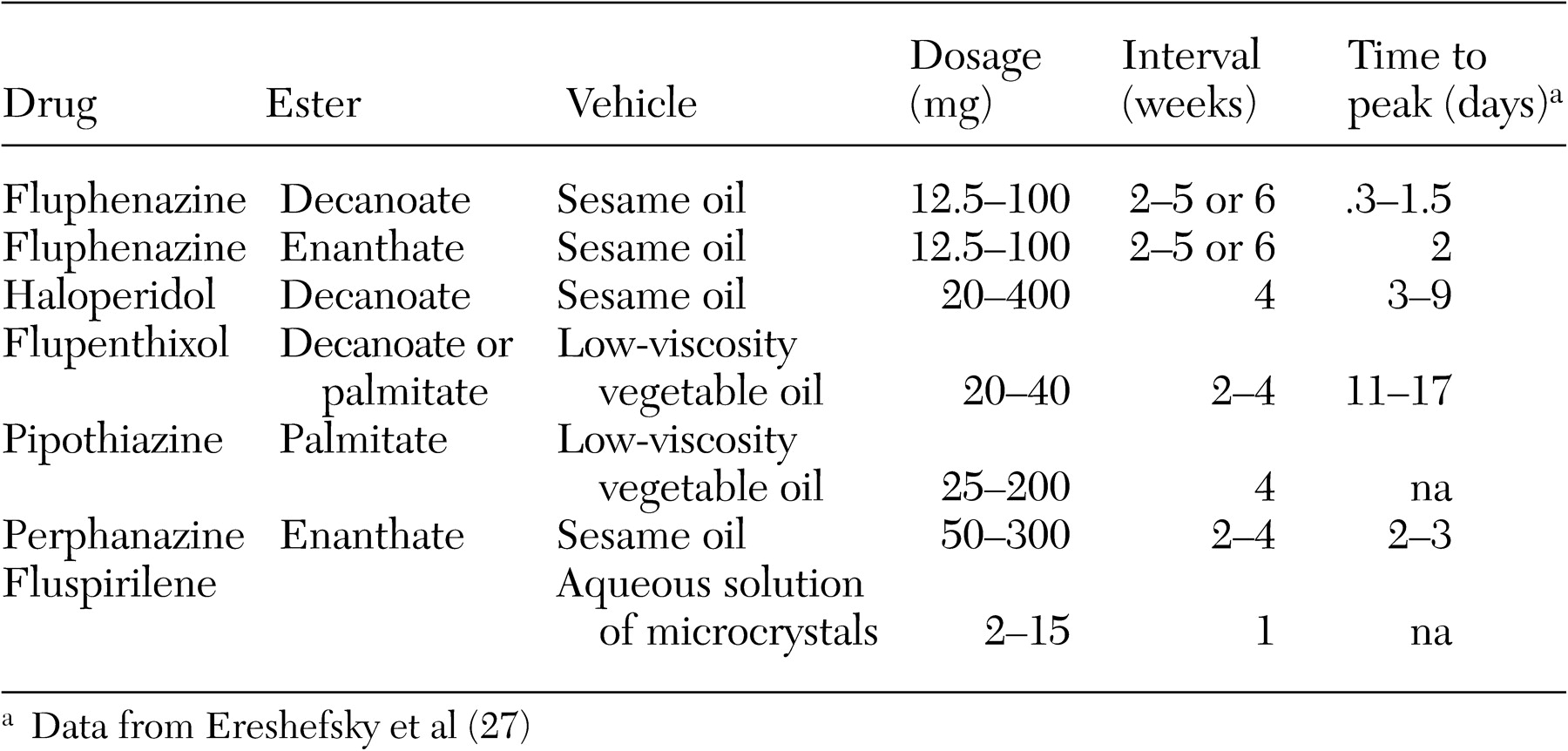
Administration and pharmacokinetics. Long-acting risperidone is administered by intramuscular injection every two weeks and contains the active drug risperidone encapsulated in polymer microspheres in an aqueous suspension (
47). Even with vigorous shaking, the particles in the suspension are not distributed in a uniform manner. Thus the entire contents of the vial must be administered to ensure delivery of the correct dosage. After administration, the microspheres are gradually hydrolyzed. Degradation of the polymer microspheres releases risperidone in a controlled manner, such that continuous, smooth, and stable plasma concentrations of drug are delivered. Single-dose pharmacokinetic studies have indicated that a small amount of risperidone (less than 1 percent) is released by diffusion at the surface of the microspheres within 24 hours of administration. This is followed by a latent period of two to three weeks, with a majority of release occurring during weeks 4 to 6 (
48).
Efficacy. Data from a 12-week double-blind placebo-controlled trial with 370 patients (
49) and a 12-month open-label study with 610 patients (
50) evaluated the efficacy and safety of long-acting risperidone among patients with schizophrenia. Treatment with long-acting risperidone has been shown to be efficacious in terms of both positive and negative symptoms of schizophrenia (assessed with use of the PANSS and the Clinical Global Improvement [CGI] scale). Long-acting risperidone was effective in multiple symptom domains, including depression and anxiety, in a sample of 110 patients with schizoaffective disorder who were followed for 12 months (
51), which suggests that its use may be associated with a reduced need for polypharmacy.
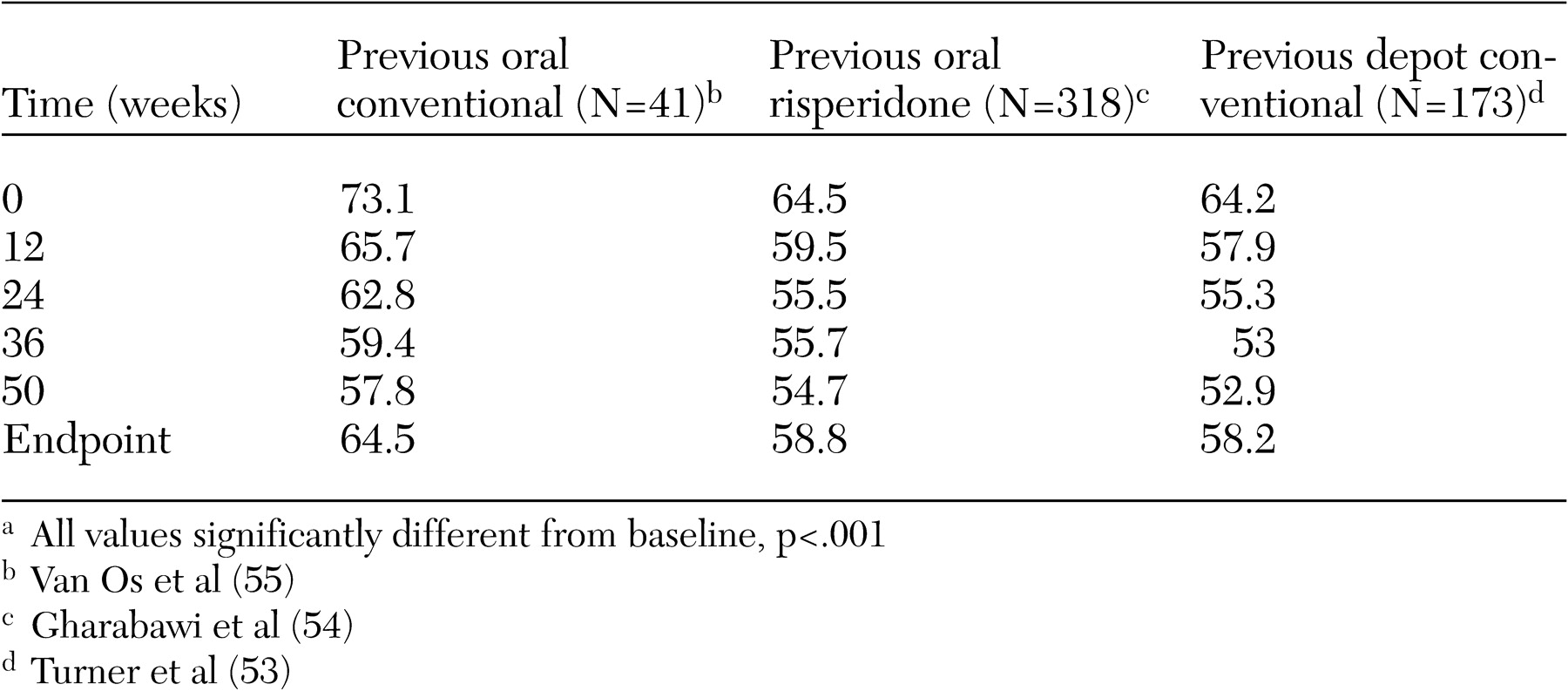
Data from the 12-month study have also shown that stable patients can safely and effectively switch from long-acting conventional therapy to long-acting risperidone (
52). Patients experienced both significant clinical improvements in PANSS and CGI scores from baseline to endpoint, despite baseline scores that indicated mild illness (
52). A separate 12-week open-label study among 166 patients who switched from conventional long-acting antipsychotic agents supported these observations (
53). Similarly, stable patients who were previously receiving oral therapy with a second-generation or conventional antipsychotic safely and effectively started therapy with long-acting risperidone (
54,
55) (
Table 2). These patients, when they switched to long-acting risperidone, experienced further significant improvements in symptoms and in the incidence and severity of extrapyramidal symptoms, with reduction of anticholinergic use.
Safety. On the basis of both clinical trial data from a total of 980 patients (
49,
50) and clinical use in real-world settings, long-acting risperidone appears to be safe and well tolerated. In the 12-week study, the incidence of extrapyramidal symptoms among patients receiving the 25 mg dosage was comparable with that of a control group receiving placebo, yet efficacy was clearly demonstrated (
49). In addition, the occurrence of extrapyramidal symptoms (as assessed by the Extrapyramidal Symptoms Rating Scale [ESRS]) was low at baseline and decreased during the 12-month study (
50). Long-acting risperidone was associated with a low annual risk of tardive dyskinesia (.68 percent) (
56), and this risk was comparable to that observed with oral risperidone (
57) and other newer oral agents, such as olanzapine (
58,
59) and quetiapine (
60). Overall, fewer than 5 percent of patients in the 12-month study discontinued treatment because of adverse events (
50). In addition, long-acting risperidone was associated with a small change in weight (.5 kg in the group receiving 25 mg of long-acting risperidone) during a 12-week trial (
49). Over the course of one year, patients receiving 25 mg of long-acting risperidone experienced an average weight gain of 1.8 kg (
50). Treatment with long-acting risperidone was associated with a reduction in hospitalization (
61); reduction in days hospitalized is one indicator of reduced relapse (
61). Furthermore, in a study of 370 patients followed for three months, many patients reported improvements in quality of life and satisfaction with their treatment (
62). In contrast with the administration of oil-based conventional long-acting antipsychotic agents, the aqueous nature of this formulation is associated with a low severity of injection-site pain (
49,
50).
Several recently published articles have reviewed the available clinical data on the pharmacokinetics, efficacy, and safety of long-acting risperidone (
22,
32,
63,
64,
65,
66). Many of these articles conclude that long-acting risperidone combines the efficacy and safety profile of a second-generation antipsychotic with the sustained delivery and assured compliance of a long-acting injectable agent. This combination provides a new opportunity to enhance disease management among patients with schizophrenia, including the treatment of clinically stable patients as well as patients who have switched from other antipsychotic agents, including long-acting conventional agents (
22,
63,
64,
65).
However, one review article did not support the view that long-acting risperidone is beneficial for persons with schizophrenia (
66). The evidence that formed the basis for the paper was the result of a search of the Cochrane Schizophrenia Group's Register (December 2002). The paper concluded that well-designed and well-reported randomized studies, firmly grounded in real-world clinical practice, are needed to fully assess the effects of this new preparation. Since 2002, a number of studies (
49,
50,
51,
52,
53,
54,
55) have been published concerning the efficacy and safety of long-acting risperidone and have helped to address the concerns raised by Hosalli and Davis (
66).
Potential candidates for treatment with long-acting risperidone
The panel of experts agreed with published clinical guidelines (
67,
68) that multiple groups of patients could be considered for treatment with long-acting risperidone. These include patients who comply poorly with their medication and those who have not responded, or have only partially responded, to their antipsychotic therapy, particularly if poor or undetermined compliance may have contributed to the lack of response. Stable patients should also be considered for treatment, because such patients have also been shown to improve when they start long-acting risperidone. It should be remembered that "stable" does not necessarily mean fully recovered without symptoms. Most patients with schizophrenia show residual symptoms or residual deficits in functioning that may benefit from a guaranteed delivery of an effective and well-tolerated antipsychotic at stable and low plasma drug concentrations.
Another important group of patients who should be considered for treatment with long-acting risperidone are those experiencing their first episode of schizophrenia. Recent clinical guidelines from the National Institute for Clinical Excellence in the United Kingdom recommend that newer antipsychotics should be considered as one of the first-choice options to treat patients with newly diagnosed schizophrenia (
45). Careful monitoring of these patients for signs of difficulty with compliance—return or exacerbation of symptoms or a lack of full recovery—may be critical in ensuring the best long-term outcome.
Many clinicians may be reluctant to initiate acute treatment with a long-acting injectable antipsychotic for a patient experiencing a first episode because of potential sensitivity to side effects and difficulty titrating to the appropriate dosage of antipsychotic. Nonetheless, early initiation of treatment with long-acting risperidone provides valuable, continuous second-generation antipsychotic coverage in this vulnerable patient group. Depending on the length of stay, initiation of long-acting risperidone in the inpatient setting may ease the transition from the hospital to the outpatient setting by ensuring that patients have the benefit of guaranteed medication delivery and their compliance status is known as they make the transition to an outpatient program.
There are a further two important reasons long-acting risperidone may be particularly useful in treating patients experiencing a first episode of schizophrenia. First, these patients are exquisitely sensitive to the effects of antipsychotics and are particularly likely to develop extrapyramidal symptoms (
69). The development of extrapyramidal symptoms early in the illness may have long-lasting negative effects on compliance and patients' perceptions of psychiatric services. The better tolerability of long-acting risperidone is likely to minimize this risk. Second, patients experiencing first episodes have very high relapse rates, with 50 percent of patients relapsing within the first 12 months of treatment (
70). Reduced relapse rates due to ensured drug delivery with long-acting risperidone are likely to substantially improve overall outcome—it has been shown that with each recurring relapse, the likelihood of symptoms returning to baseline levels decreases (
71,
72). Therefore, treatment with long-acting risperidone at the earliest stages of schizophrenia should reduce the likelihood of more severe symptoms (
73), thus improving long-term outcomes (
74). Furthermore, by treating patients early, when their social and vocational support networks are still intact, the likelihood of maintaining these critical building blocks of quality of life is maximized.
Initiating treatment
The panel of experts proposed several treatment recommendations for initiating therapy with long-acting risperidone, which concurred with published clinical guidelines (
32,
67,
68). These recommendations, outlined below, will help to ensure that safe, effective, and confident initiation of long-acting risperidone is achieved when patients switch from other treatments. To minimize risks and maximize outcomes, it is recommended that initiation of long-acting risperidone be carefully tailored to individual patients. As with any new therapy or therapy switch, potential changes in mental state and functioning should be discussed with patients and caregivers.
Among patients who have had no exposure to oral risperidone, it is recommended that treatment be preceded by a "hypersensitivity challenge" with a single test dose (personal communication, Eerdekens M, 2004). After the first injection, patients should also receive additional antipsychotic coverage for the three weeks following the first injection to ensure that adequate antipsychotic is afforded until the main release phase has begun (
75). The nature of the additional antipsychotic coverage will depend on the previous antipsychotic therapy.
Long-acting risperidone should be initiated at the recommended starting dosage of 25 mg every two weeks. The clinician should carefully consider the possibility that the prescribed dosage of oral medication might be quite different from the consumed dosage. Relatively high prescribed oral dosages may have been purposely or inadvertently reduced by a patient. Therefore, starting such a patient on a dosage higher than 25 mg of long-acting risperidone may expose the patient to much more active antipsychotic than he or she has taken previously, leading to potential adverse events and rejection of the medication by the patient. The 25 mg dosage is effective for most patients and is associated with a side effect profile similar to that of placebo (
49). Dosage strengths of 37.5 and 50 mg are also available.
Because the treatment of schizophrenia is a long-term process, both patients and treatment teams should be advised to allow time for the full benefits of treatment with long-acting risperidone to emerge—not only resolution of symptoms and the prevention of relapse but also improvements in functioning and motivation. Clinicians have recognized the value of avoiding side effects on initiation of therapy to set the stage for long-term cooperation. Few things are more likely to reduce patient cooperation with a treatment regimen than the acute onset of unanticipated and frightening side effects. Thus, starting the patient on a dosage that is unlikely to produce side effects is particularly important for the long term. The sensitivity to medication seen among patients experiencing an early episode of schizophrenia means that this group is in particular need of initiation with the lowest possible dosage.
Adjunctive therapy with agents other than antipsychotics should be considered on a case-by-case basis. If the previously used antipsychotic therapy was sedating, a hypnotic can be of value in the early stages of treatment with long-acting risperidone to control symptoms such as insomnia. The patient should adjust to the less sedating effect of long-acting risperidone quickly, and the adjunctive hypnotic should be discontinued at that point. A more suitable approach is to advise patients and their caregivers in advance of the reduction in sedation and to inform them of the changes that they are likely to experience when switching from their current treatment. This is because many patients will have undergone long periods of passivity and demotivation. The use of hypnotics should be restricted to individual clinically warranted cases.
Among patients who have previously been treated with oral antipsychotic agents and for whom adjunctive anticholinergic medication may have been prescribed to control extrapyramidal symptoms, anticholinergic treatment should be continued, at least until the next injection, after cessation of oral supplementation therapy. At this point, the patient should be consulted about his or her experience of extrapyramidal symptoms. If no extrapyramidal symptoms are present, the dosage of anticholinergic should be halved. If, at the next injection—for example, two weeks later—extrapyramidal symptoms are still not present, the anticholinergic agent can be discontinued with a high likelihood of success. In the 12-month study of long-acting risperidone, very few patients required the initiation of antiparkinsonian therapy (
50).
Different recommendations apply to patients who have previously been treated with long-acting conventional agents and concomitant anticholinergic therapy. Anticholinergic therapy should be continued for three or four months to ensure a minimal risk of dystonic reactions from the remaining depot antipsychotic. Extrapyramidal symptoms should be evaluated carefully before discontinuation of the anticholinergic agent is considered. If the patient does not experience any extrapyramidal symptoms, the dosage of the anticholinergic agent can be tapered gradually, over a four- to six-week period. If extrapyramidal symptoms reemerge during that time, the dosage of anticholinergic agent should be increased and tapered off more gradually. Anticholinergic agents should be used only when extrapyramidal symptoms are present, not as prophylaxis.
Maintaining therapy safely
Steady-state levels of risperidone are reached after the fourth dose of long-acting risperidone—six to eight weeks after initiation of therapy. Dosage increases should be avoided during the first six to ten weeks, and both the patient and the physician should exercise patience during this period. Certainly, increasing the dosage more frequently than every four weeks is contraindicated, because therapeutic benefit from the long-acting risperidone will not be seen before a minimum of three weeks. If patients experience transient symptom breakthrough, oral supplementation with an antipsychotic may be given. The temptation to use high dosages and to rapidly escalate dosages possibly stems from misguided use of the occurrence of extrapyramidal symptoms as an indicator of dopamine receptor blockade, which is considered a clinical indicator of sufficient medication with conventional antipsychotics. With the availability of the second-generation antipsychotics, particularly long-acting risperidone, it is no longer necessary to use the emergence of extrapyramidal symptoms as an indicator of efficacy.
Given the pharmacokinetic profile of long-acting risperidone, it is important to remember that, when the dosage is changed, there will be a lag period of three weeks before the benefits can be observed; additional oral antipsychotic coverage may be required during this period (
75). The recommended dosage of long-acting risperidone is 25 mg every two weeks, The maximum dosage of 50 mg should not be exceeded; data from the clinical trials showed that a 75 mg dose did not confer any clinical benefits over the 50 mg dose and was associated with higher levels of extrapyramidal symptoms (
49,
50).
To ensure optimal efficacy and safety, long-acting risperidone should continue to be administered every two weeks. If a dose is missed in the steady-state phase and the last administration was less than four weeks previously, the next dose can be given as soon as possible. This approach should provide sufficient antipsychotic maintenance, although the patient should be monitored carefully for breakthrough symptoms. However, if more than six weeks have passed since the last dose, or steady-state levels have not yet been reached, the safest way to continue treatment is to administer long-acting risperidone as though treatment were being initiated afresh, supplementing with oral antipsychotic for the first three weeks, to enable therapeutic levels to be established once again.