A few well-controlled clinical trials with limited sample sizes have suggested that the opiate antagonist naltrexone is an efficacious adjunctive medication in the treatment of alcoholism (
1,
2). Volpicelli and colleagues (
1) treated 70 male alcoholics with 50 mg/day of naltrexone or placebo for 12 weeks while they received a multimodal “standard rehabilitation treatment.” The naltrexone-treated individuals had a reduced relapse rate, a lower overall number of drinking days, and a reduction of craving in comparison with the placebo-treated individuals. Generalization from this trial was limited, since subjects received a relatively intensive psychosocial intervention during the early at-risk relapse period, which likely aided in medication compliance.
O’Malley and colleagues (
2) randomly assigned male and female alcoholics, who were primarily self-referred for outpatient treatment, to receive 50 mg/day of naltrexone or placebo and to concomitantly receive either a coping skills therapy or a therapy supportive of abstinence. Naltrexone decreased relapse rates, the percentage of drinking days, and the total number of drinks during the study in comparison with placebo, irrespective of the type of therapy received. In addition, there appeared to be an interaction between therapy and medication such that individuals treated with naltrexone and supportive therapy had greater continuous abstinence than the other treatment groups, while coping skills therapy appeared to reduce the relapse rate for the subjects who consumed alcohol (“slip” drinking) during the treatment evaluation. Reduction in craving was evidenced most strongly in the subjects who received naltrexone and coping skills therapy and who completed the trial.
Subsequently, Volpicelli and colleagues found that compliance with medication played a crucial role in the observed increased efficacy of naltrexone over placebo (
3). In that study, 97 male and female alcoholics who had just completed a medical detoxification for alcohol withdrawal received either 50 mg of naltrexone or placebo daily plus relapse prevention therapy. Even though the dropout rate in that study group was only 27%, the main effects of naltrexone (less relapse, fewer drinking days) could only be found in those individuals who, according to pill counts, had taken over 90% of their medication. Of interest, although craving was assessed as in the previous study (
1), there was no significant difference in craving between the naltrexone-treated and the placebo-treated subjects in either the intent-to-treat group or the maximally compliant group.
Naltrexone is reported to reduce the high after alcohol consumption (
4) and to decrease the level of intoxication and “incentive to drink” after slip drinking (
5). Naltrexone-mediated reduction of craving and incentive to drink, although not consistently reported, does have some similarity to findings in animals which suggest that opiate antagonist pretreatment leads to a lower incentive or motivation to consume alcohol (
6,
7).
Cognitive behavioral therapy has been shown to be an effective treatment for alcoholism (
8) and was one of the therapies used during Project MATCH, a large multisite study of 1,726 alcoholics that attempted to match alcoholic characteristics to therapy response (
9). In that study, in which our group participated, manual-guided cognitive behavioral therapy (
10) was found to lead to a marked reduction in both drinking days and drinks per drinking occasion for up to 15 months after the initiation of treatment. It was reasoned that cognitive behavioral therapy, which addresses issues of craving, management of slip drinking, reduction of relapses, and other similar techniques would be particularly amenable to augmentation with naltrexone as suggested previously (
2).
The goal of the present study was to replicate and extend the reported data on the efficacy of naltrexone in the treatment of alcohol dependence (
1,
2,
11). To maximize the internal validity of this study, particular attention was given to study group size, subject selection, measurement of compliance (
12), craving (
13,
14), biological markers (
15), and the use of a manual-guided cognitive behavioral therapy approach (
10). This report presents the findings in a randomized, double-blind 12-week trial of the efficacy of naltrexone or placebo added to cognitive behavioral therapy for outpatient alcoholics.
Method
The study subjects were persons seeking outpatient treatment for alcoholism who were either referred to our clinical service or responded to advertisements for the research study. Approximately 1,094 individuals were screened over the telephone, and 440 were invited for in-person screening. Of these, 338 were screened in person, 190 gave written informed consent, and 132 entered the study. The inclusion criteria were 1) age 21–65 years, 2) meeting the DSM-III-R criteria for alcohol dependence, including criterion 2 (loss of control over drinking), 3) consumption, on average, of five or more drinks per day in the last 30 days, 4) residence within 1 hour’s drive of the clinic, 5) a stable living situation and availability of a collateral reporter, and 6) ability to maintain sobriety for at least 5 days before study entry (during the evaluation period). The exclusion criteria were 1) a previous inpatient detoxification in which medication was taken, 2) other current drug abuse or dependence (including marijuana), 3) ever having abused opiates, 4) a current major psychiatric disorder as determined by the Structured Clinical Interview for DSM-III-R (SCID [
16]), 5) a serious or unstable medical condition, 6) current use of psychotropic or antiseizure medications or disulfiram, 7) pending legal charges except for driving while intoxicated, and 8) liver function test results (alanine aminotransferase and aspartate aminotransferase) greater than 2.5 times normal.
Reasons for nonparticipation (N=206) among the 338 individuals screened in person included the following: 55 (27%) chose not to participate, 42 (20%) did not meet the alcohol use or 5-day sobriety criteria, 39 (19%) had other psychiatric diagnoses, 20 (10%) had other substance abuse, 17 (8%) had exclusionary medical conditions, 17 (8%) lacked social stability, and 16 (7%) had had previous inpatient treatment or treatment with naltrexone.
Of the 132 subjects randomly assigned to treatment, 131 returned for at least one evaluation visit and were considered evaluable for the intent-to-treat analysis.
After initial screening and a complete description of the study to the subject, written informed consent according to the guidelines of our institutional review board was obtained. Subjects were then assessed over a 5- to 10-day period during which they returned to the clinic on at least three occasions. On these visits they had to show evidence, by verbal report (self and a collateral reporter) and a Breathalyzer test, of having maintained abstinence for at least 5 consecutive days before random assignment to a study condition. During this assessment period, the following were administered: the SCID (
16), the Addiction Severity Index (
17), the Alcohol Dependence Scale (
18), the Obsessive Compulsive Drinking Scale (
13,
14), four analog scales measuring craving (amount, duration, frequency, and intensity), and the Form 90 calendar method for charting daily drinking, drug use, and service utilization (
19). Subjects were instructed to complete the Obsessive Compulsive Drinking Scale and analog craving scales for the last week of active drinking before study entry. Blood was obtained for general health screening, liver function tests, and measurement of the alcohol use markers γ-glutamyltransferase and carbohydrate-deficient transferrin (CDTect; Avis-Shield, Oslo). Urine for screening for illicit drugs was also obtained. All subjects underwent a physical examination and provided a review of past and current medical symptoms and conditions. Collateral informants were contacted to verify alcohol consumption, general health, and other drug use information.
Subjects were randomly assigned to receive either naltrexone, 50 mg, or an identical-appearing placebo capsule daily for 12 weeks (84 days). Each capsule also contained 100 mg of riboflavin, added in order to ascertain quantitative weekly urinary riboflavin levels, which were measured by fluorescence assay (
13) at the end of the study. All subjects were required to attend 12 weekly sessions of individual manual-guided cognitive behavioral therapy (
12). The therapists were supervised by one of us (L.R.W.), who reviewed cases and ensured quality control over the delivery of the manual-guided therapy. Emergency sessions and/or spousal attendance could be allowed on a maximum of two occasions, as directed by the manual.
Subjects were seen by the research assistant weekly for outcome assessment and by study physicians at the end of weeks 1–4, 8, and 12 for reports of adverse events, physical evaluation, and medication checks. Weekly assessments included the timeline follow-back calendar method for daily estimation of drinking (
20), the Obsessive Compulsive Drinking Scale, the analog craving scales, and a physical symptom checklist. The assessments obtained at baseline were repeated at the end of week 12 or at study termination. Blood for determining liver function and γ-glutamyltransferase and carbohydrate-deficient transferrin levels was obtained at the end of study weeks 4, 8, and 12. If a subject terminated earlier than 12 weeks, a “reason for termination” checklist was filled out with the use of all data available to the research group and the study therapists. If a subject terminated early, every attempt was made to gather week-12 data in order to have a continuous record of drinking over the course of the study and to obtain end point measures. All but two subjects had week-12 data collection, leading to an end point data collection rate of 98.5%.
Baseline variables were examined for differences between groups with the use of analysis of variance or chi-square tests where appropriate (SPSS analytic package [
21]). All outcome analyses were conducted under an intent-to-treat analysis plan. Time-to-relapse survival analyses used the Kaplan-Meier statistic. Group outcome differences in percentage of days abstinent and drinks per drinking day were analyzed by an analysis of covariance (ANCOVA) with the baseline measures of these variables as covariates. The biological drinking markers, carbohydrate-deficient transferrin and γ-glutamyltransferase levels, were evaluated by both repeated measures and end point ANCOVA with baseline levels as covariates.
Group differences on the analog craving scales and the Obsessive Compulsive Drinking Scale and its factors were analyzed by repeated measures ANCOVA with baseline values on the respective scales used as covariates. Analog craving measures were averaged, and the mean for each subject at each time point was used in the analysis. In a similar fashion, the Obsessive Compulsive Drinking Scale, when previously factor analyzed (
22), showed a three-factor solution that was better than the two-factor subscale discrimination previously described (
13,
14). Therefore, the focus of the analysis was on three factors: obsessive thinking about drinking (items 1, 2, 4, 11, and 13), resistance/control impairment factor (items 5–8, 12, and 14), and social/work interference caused by drinking (items 3, 9, and 10).
Results
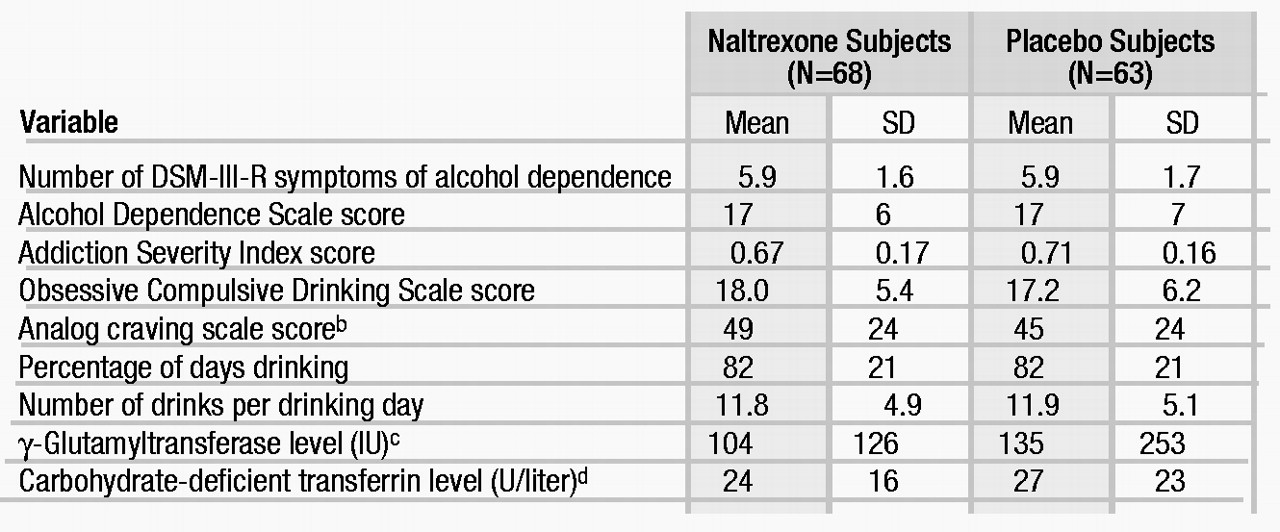
The salient demographic variables for the 131 randomly assigned subjects with data that could be evaluated were—for the naltrexone-treated group (N=68) and the placebo-treated group (N=63), respectively: age (mean=41 years, SD=10, and mean=44 years, SD=10), male gender (69% and 73%), Caucasian race (89% and 82%), married (66% and 70%), employed full time (81% and 81%), and education (mean=14 years, SD=3, and mean=14 years, SD=3). There were no significant differences between the two groups on any variable. In general, the individuals in this study were well-educated, employed, married, and socially stable. There was no significant difference in any measure of severity of alcoholism between the two treatment groups (table 1).
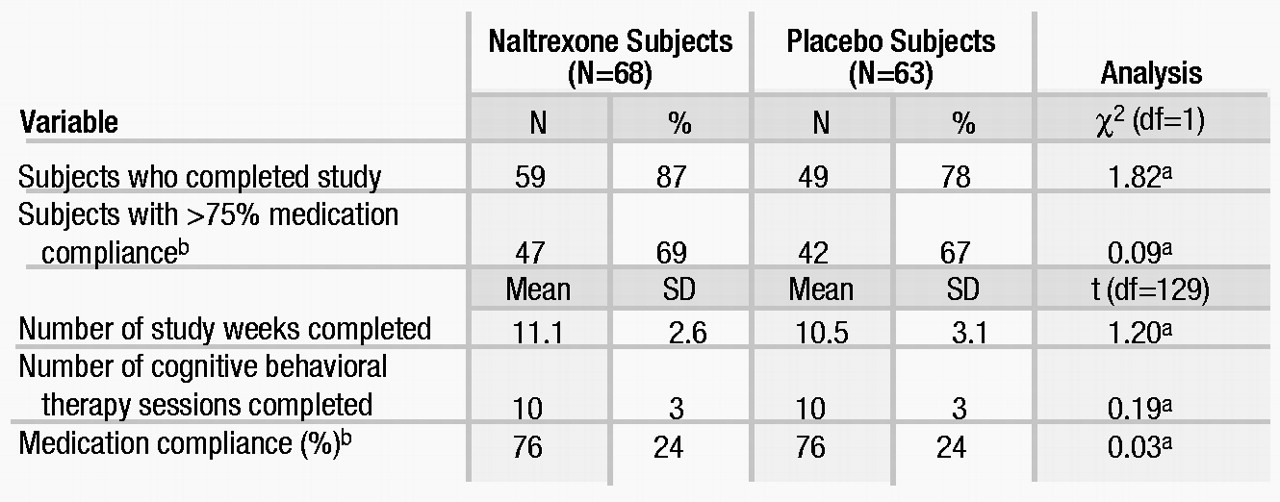
The important indicators of this study’s internal validity are given in table 2. There was no difference between the groups in any of these indicators. Overall, 83% (N=108) of the subjects completed the study, with an average of 11 of the 12 study weeks completed and about 10 of the 12 therapy sessions attended. In general, these indicators suggest that in comparison with other alcohol treatment outcome studies, retention and compliance were as good as, if not better than, could be expected.
Twenty-three subjects (nine in the naltrexone group and 14 in the placebo group) terminated the study early. Reasons for early termination included clinical deterioration (one naltrexone and two placebo subjects) and adverse events in one naltrexone subject (abdominal distress) and one placebo subject (sexual dysfunction). In addition, seven naltrexone and 11 placebo subjects missed two consecutive appointments (refused treatment or were lost to follow-up). There were no significant differences between the naltrexone and placebo groups in the number of subjects terminating prematurely or in the reasons for termination.
The primary dependent variables for drinking outcome evaluation were selected before the study began. They were based on the variables used in other investigations of naltrexone (
1,
2) and on the recently completed large-scale, multisite, psychotherapy-matching outcome study Project MATCH (
9). These variables were time to first relapse (defined as five or more drinks per day for male subjects and four or more for female subjects), percentage of days abstinent, and drinks per drinking day over the 84 days of the study.
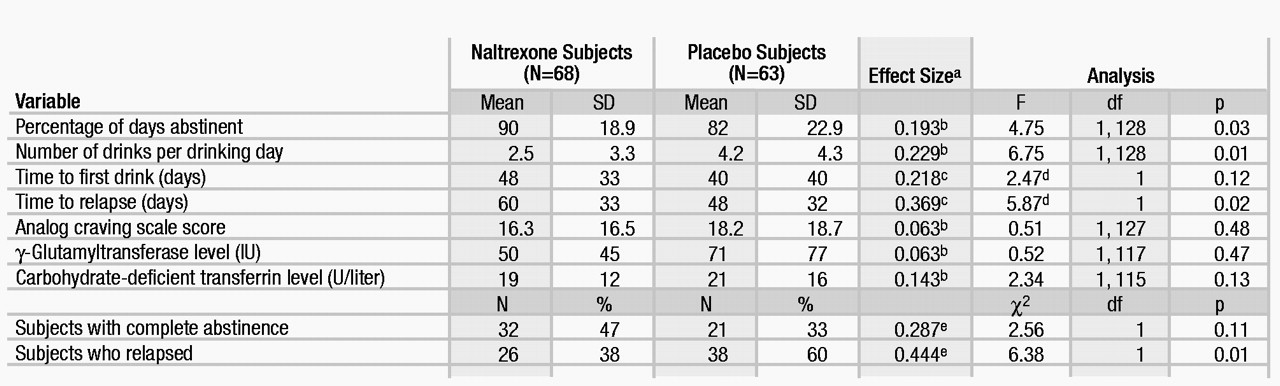
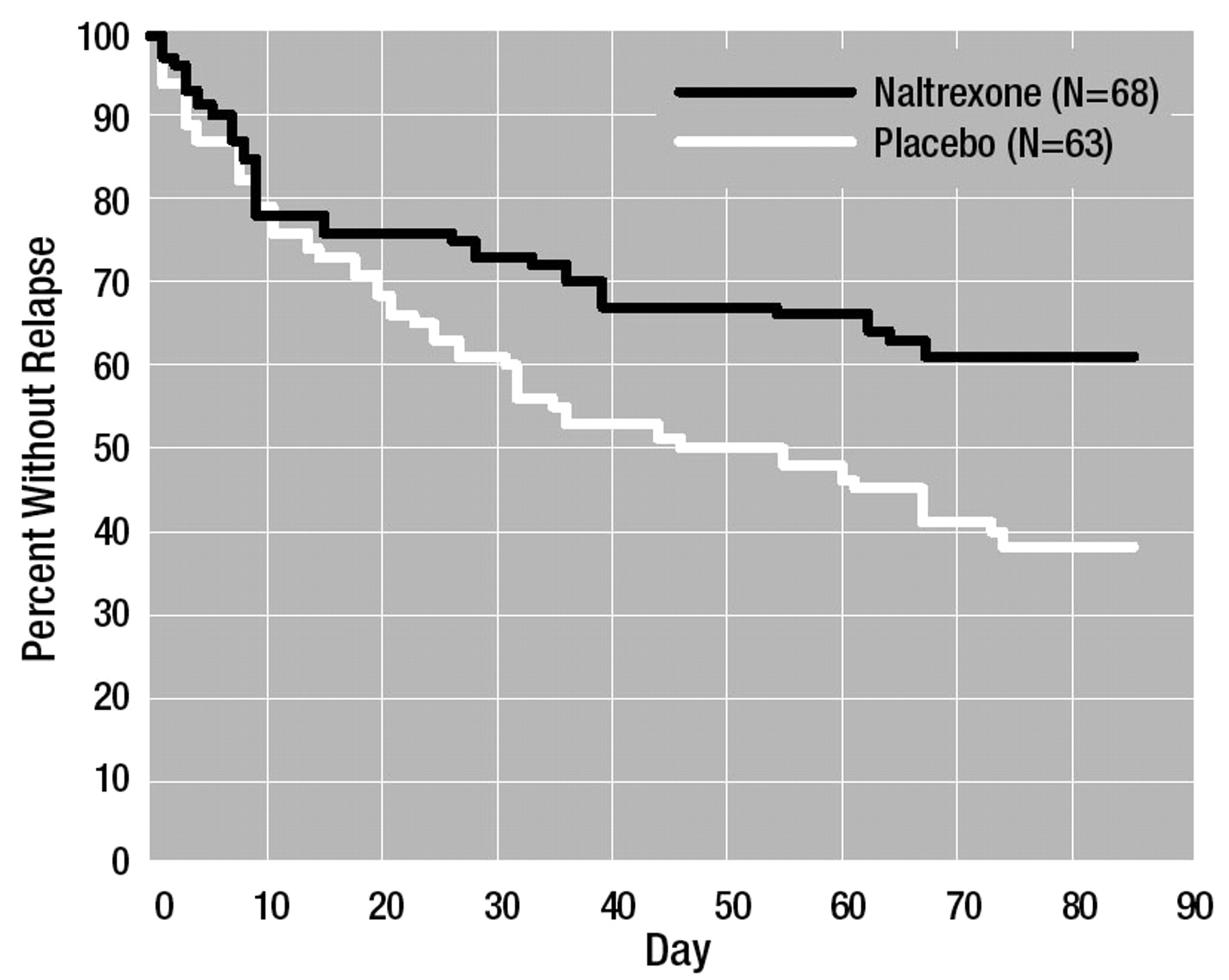
The survival curve of time to first relapse is shown in figure 1. Overall, the survival function for the subjects treated with naltrexone was significantly better than that of the subjects treated with placebo. At the end of the study, 62% (N=42) of the naltrexone subjects had not relapsed, compared with 40% (N=25) of the placebo-treated subjects. Table 3 provides other alcohol consumption data. The subjects treated with naltrexone had a significantly higher percentage of time abstinent and, when they did drink, had significantly fewer drinks per drinking day than the placebo-treated subjects.
To provide a more complete analysis of the effect of naltrexone on alcohol drinking behavior, we performed some secondary analyses on salient alcohol consumption variables. Although the naltrexone group had an overall better survival function for time to first day of any drinking (one or more drinks) (mean time to first drink was 60 days for the naltrexone subjects and 22 days for the placebo subjects), this was not statistically better than that of the placebo group (Kaplan-Meier log-rank=2.47, df=1, p=0.12); by the end of the treatment period, 47% of the naltrexone group and 33% of the placebo group had maintained continuous abstinence. However, on average, placebo subjects who relapsed had only 6 days (SD=7) to the next heavy drinking day, whereas the naltrexone subjects had 14 days (SD=18) until the next heavy drinking day (t=2.40, df=62, p<0.05).
The blood markers carbohydrate-deficient transferrin and γ-glutamyltransferase provide data on subjects’ drinking that are independent of verbal report. A repeated measures (weeks 4, 8, and 12) ANCOVA with baseline level as the covariate showed a main effect of time for carbohydrate-deficient transferrin (F=11.86, df=2, 236, p<0.0001) and for γ-glutamyltransferase (F=43.24, df=2, 240, p=0.0001) but no group or group-by-time differences. Both carbohydrate-deficient transferrin and γ-glutamyltransferase had significantly decreased over prestudy levels, but this decrease was similar in the two groups, as indicated by the end point levels in table 3.
There was no difference between the medication groups on the composite analog craving measure (table 3). However, the total Obsessive Compulsive Drinking Scale scores were lower over the course of the study in the naltrexone group than in the placebo group (F=3.30, df=1, 128, p=0.07). Of the three Obsessive Compulsive Drinking Scale factor scores, the resistance/control impairment factor score was significantly lower in the naltrexone group than in the placebo group (for the total factor score, F=4.58, df=1, 128, p=0.03; when the two drinking items were removed, F=6.13, df=1, 128, p<0.02), while the other factors (obsessive thinking and work/social interference) were not significantly different between the treatment groups.
All study subjects filled out a physical complaint questionnaire weekly. The following complaints were rated by physicians as possibly or probably related to the study drug: nausea/vomiting (14% of the placebo subjects and 34% of the naltrexone subjects; χ2=6.76, df=1, p<0.01), abdominal pain (11% of the placebo subjects and 31% of the naltrexone subjects; χ2=7.60, df=1, p<0.01), daytime sleepiness (27% of the placebo subjects and 46% of the naltrexone subjects; χ2=4.88, df=1, p<0.05), and nasal congestion (25% of the placebo subjects and 46% of the naltrexone subjects; χ2=5.80, df=1, p<0.05). Only one subject from each group dropped out of the study because of a stated adverse drug effect (the naltrexone subject with abdominal discomfort and the placebo subject with sexual dysfunction).
It should be noted that while four gender-specific questions about sexual desire and performance were asked, there was no difference between the placebo and naltrexone groups on any of these items.
Discussion
The selection of the study group, the study design, and possibly the nature of the cognitive behavioral therapy all led to high retention, completion, and compliance in both treatment groups. The study group of 131 subjects was larger than groups in previously conducted trials. This, combined with a low level of missing data and high internal validity, led to sufficient statistical power for determining differences between groups. Overall, the results of this trial support the observations made in earlier trials with fewer subjects (
1,
2) and suggest that the results of a subsequent trial (
3), which found medication group differences only in compliant subjects but not in the intent-to-treat analysis, may have been due to a type II error.
Consistent with previously reported studies, naltrexone-treated individuals had fewer drinking days (
2) and fewer drinks per drinking day (
1,
2). In addition, naltrexone reduced the rate of relapse into heavy drinking (defined consistently in these studies as five or more drinks per day for men and four or more for women). It is less clear whether naltrexone can affect the ingestion of the first drink (either time to first drink or total abstinence rate) and also less clear what happens once a person has a defined relapse day. In our study, although naltrexone led to a longer time to first drink (median=60 days for naltrexone subjects and median=22 days for placebo subjects), this difference was not statistically significant. Looked at in another way, 47% of the naltrexone subjects were completely abstinent throughout the trial, compared with 33% of the placebo subjects, which although not statistically significant, suggests an effect of naltrexone on the maintenance of abstinence. It must be remembered, however, that cognitive behavioral therapy, at least as it was used in this study and in Project MATCH (
10,
12), does not demand abstinence as a firm goal of treatment. This may account for the discrepancy in naltrexone’s effects on full abstinence between the cognitive behavioral therapy used in this study and the supportive abstinence-based therapy used in one arm of the O’Malley et al. study (
2). In that regard, the cognitive behavioral therapy of this study and the coping skills therapy of the O’Malley et al. study, when used in conjunction with naltrexone, had similar effects on relapse reduction. This is not surprising, since the two types of therapy share a common theoretical framework, focus on the same goals (reduction of alcohol relapse), and use similar therapeutic techniques.
Our analysis of the time between a first relapse (or heavy drinking day) and a second relapse (or heavy drinking day) in the naltrexone-treated group compared with the placebo-treated group is of interest. Naltrexone subjects had, on average, about twice the time between first and second relapse episodes (14 days versus 6 days). This finding is consistent with the concept that naltrexone allows the alcoholic to keep at least partial control over alcohol consumption after a slip drinking episode. Since it has been suggested (
3,
5) that this increase in control may occur because of the reduced reinforcement of alcohol after a slip, this effect may be particularly important for the combination of naltrexone and cognitive behavioral therapy. The theoretical basis of cognitive behavioral therapy is that the individual should try to use cognitive behavior strategies to reduce the chance that an alcohol slip may turn into a relapse. The fact that naltrexone may reinforce this construct is heuristically appealing and could have great practical treatment implications.
Although naltrexone has been called an anticraving drug (
25), its role in the reduction of craving—a concept that is elusive and hard to define (
26,
27)—is still unresolved. As noted previously, the data regarding the effect of naltrexone on craving have been inconsistent. In the first study by Volpicelli and colleagues (
1), a positive effect of naltrexone on craving was noted, but in the second study by this group (
3), in which a similar analog rating scale was used, there was no effect noted, even in the most compliant subjects. O’Malley and colleagues (
2), on the other hand, noted that the level of craving (as measured by an analog scale) was lower in the subjects taking naltrexone who also received coping skills therapy but was higher in the subjects taking naltrexone who received supportive therapy than in placebo control subjects. In subsequent analyses (
5), it was found that craving after an alcohol slip was lower in naltrexone-treated subjects and, furthermore, that a higher level of baseline craving was predictive of better response to naltrexone treatment (
28). Some of the inconsistency in these studies may have derived from the craving measure that was used. Both studies used a one-item analog scale that is highly subjective and likely to be highly variable.
The self-rated Obsessive Compulsive Drinking Scale was developed by our group to provide an instrument with improved psychometric qualities and face validity to measure some cognitive and behavioral aspects of craving (
12,
13). While the average of the four analog craving measure scores was not different over the course of this study in the naltrexone and placebo groups, the total Obsessive Compulsive Drinking Scale score tended to decrease more in the naltrexone group than in the placebo group. Of interest, the resistance/control impairment factor of the scale (even with the two quantity/frequency drinking items removed) was significantly improved in the naltrexone group (
22). While needing replication, this finding supports the hypothesis that reduced drinking in naltrexone-treated individuals is related to more resistance to alcohol-related thoughts, urges, and behavior, with greater control being exercised over these aspects of craving.
Although this study had very high internal validity, the generalizability (external validity) of the findings reported here may be limited. It must be remembered that the subjects selected for this study were persons whose alcohol dependence was not very severe, who were not particularly treatment-resistant, and who were socially stable, reasonably motivated, and had no comorbid current other drug abuse or severe psychiatric problems. This subject population was selected to minimize the type II error rate. Despite this, the superiority of the effects of naltrexone over those of placebo, when added to cognitive behavioral therapy, was modest (effect sizes=0.2–0.5). While a number of alcohol-dependent patients seen in primary care practice, psychiatric outpatient settings, and substance abuse referral centers may fit the profile of our study participants, many do not. Only more well-controlled clinical trials examining different alcoholic populations can address the effectiveness of naltrexone across the alcohol dependence spectrum.