THE CASE
Mr. W's wife and children have noticed his decreasing memory over the past year. This 66-year-old working executive is completely functional other than making lists when he goes shopping, but he is starting to worry. “He forgets details of conversations we have,” his wife complains. His medical history is unremarkable except for hypertension, for which he takes ramipril and hydrochlorothiazide. He also takes 80 mg of acetylsalicylic acid daily, and diazepam for sleep on a regular basis. He gets little exercise, but he is in a challenging and enjoyable position at work. Findings on physical examination are unremarkable. The results of the neurologic examination are normal aside from cognition. On brief cognitive testing with the Mini-Mental State Examination, Mr. W has a score of 27 out of 30, he is oriented, and his concentration (serial 7 subtrations) is normal. He copies a pentagon well. His delayed verbal memory, however, is quite impaired, and he recalls only 1 of 3 words in a list after a 1-minute delay. He asks you if this is the beginning of Alzheimer disease, whether he should retire and what he can do to prevent any further deterioration. You suspect he meets the criteria for mild cognitive impairment. What investigations and treatment should be initiated?
Between 25% and 75% of elderly people report that their memory is worse than it was when they were younger, depending on how the question is phrased. (
1,
2) Almost all of these people worry that this change in memory might represent the beginning of Alzheimer disease or another type of dementia. Indeed, public opinion polls have shown that concerns about Alzheimer disease are among the 3 leading worries of elderly people. (
3) In the past, physicians tended to offer blanket reassurances that “you are normal.” Although the majority of elderly people who note memory changes will not go on to have dementia, dementia does develop in 8% of elderly people, (
4) so such reassurances may not be appropriate.
In this article, we focus on the “grey zone” between no dementia and dementia. We provide physicians with practical guidance on the definition, diagnosis and treatment of mild cognitive impairment and cognitive impairment with no dementia, based on recommendations from the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (
Box 1). A description of the process used to generate the recommendations is provided in the first article of the series (
6) and in an online appendix accompanying this article (available at
www.cmaj.ca/cgi/content/full/178/10/1273/DC2). Published background papers provide details of the evidence-based reviews on which the recommendations are based. (
7,
8)
THE CONCEPT OF MILD COGNITIVE IMPAIRMENT
Using the accepted published criteria, (
6,
9,
10) most physicians feel fairly confident now in diagnosing Alzheimer disease and other types of dementia. Patients who do not have dementia but who may not be “normal” remain diagnostic challenges. In fact, the concept of “normality” in an elderly person is itself controversial. Although most elderly people note subtle changes in their memory and cognition since youth, most feel that their memory performance and daily functioning is similar to that of others their age. The majority of experts view this as normal “cognitive aging” and that normality must be determined with respect to a particular age group. (
11–
13) This concept of normality does not require the total absence of unrelated diseases (e.g., in a survey of 2590 adults in the United States, 19% of men and 23% of women aged 65 years or more reported taking at least 5 prescription medications (
14) but, rather, performance at an equivalent level to their peer group.
In the large, population-based Canadian Study of Health and Aging, elderly people were classified as having one of the following conditions: no cognitive loss; cognitive impairment, no dementia; or dementia. (
4,
15) The study defined cognitive impairment, no dementia, as the presence of objective cognitive impairment in any domain tested, with performance falling between the 2 poles of normality and dementia. Overall, 16.8% of elderly people were found to fall into this category. (
16,
17) These individuals showed an increased risk of eventual dementia (about 50% after a 5-year follow-up period) and death. (
18)
The label “cognitive impairment, no dementia,” has the benefit of requiring physicians to simply conclude that there is cognitive impairment, without having to consider what the degree of decline is, whether functional impairment is present or what the underlying causes might be. A number of limitations, however, are inherent in using this term. One drawback is that a person might be considered as having cognitive impairment, no dementia, because of memory loss due to an underlying condition (e.g., lifelong static brain damage, mental retardation or schizophrenia) that would be obvious to the treating physician or specialist. In this case, the prognosis and approach to the diagnosis of cognitive impairment, no dementia, would not be as relevant to the treating family physician or specialist, who is focused instead on the patient's complaint of a new decline in memory. When the label was applied in a cohort of patients referred to memory clinics in Canada, the causes appeared quite heterogeneous, (
19) including amnestic, vascular, psychiatric, neurologic, metabolic and mixed.
A more widespread term currently used to characterize this grey zone between normality and dementia is “mild cognitive impairment.” (
20–
22) This clinical label is applied to elderly people with short- or long-term memory impairment who have no significant daily functional disability. The initial criteria for mild cognitive impairment requires a subjective report of cognitive decline from a former level, gradual in onset, and present for at least 6 months. This subjective report is supplemented by objective evidence of decline in memory and learning on brief or extensive cognitive testing. Other cognitive domains remain generally intact. (
23) There is no clear delineation as to how the presence of memory loss is to be established, but the presence of objective signs is emphasized. Later work enlarged the definition so that other domains besides memory might show impairment. In all cases, this term excludes people who have significant depression, delirium, mental retardation or other psychiatric disorders that are likely responsible for the impairment. If the memory loss is severe and accompanied by significant functional impairment and other cognitive impairments, the patient meets the clinical criteria for dementia, not mild cognitive impairment.
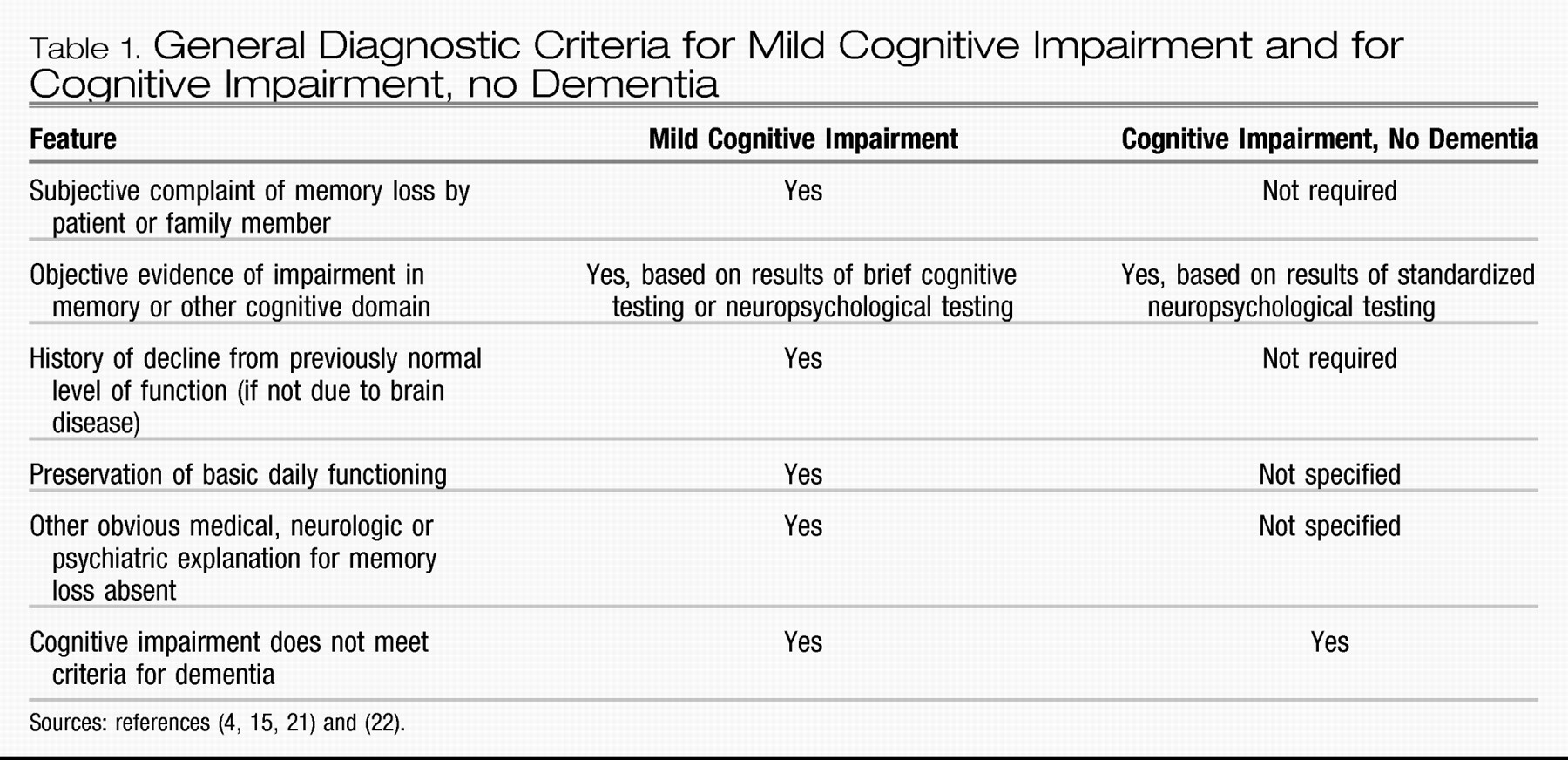
The concept of mild cognitive impairment is intended to capture and classify patients like Mr. W, described at the beginning of the article. He seems to have a cognitive problem that one would be loathe to label as “normal,” and yet it is not severe enough to qualify as dementia. The differences in diagnostic criteria between mild cognitive impairment and cognitive impairment, no dementia, are provided in
Table 1.
VASCULAR FACTORS
Many people with mild cognitive impairment have vascular risk factors. In some cases, imaging reveals silent cerebral infarcts that amplify the effects of any degenerative brain disease. In others, the patient may have had a series of silent or clinical strokes that have a cumulative effect on their cognition, and the brain shows none of the changes indicative of Alzheimer disease. In such cases, we might use the term “vascular cognitive impairment” rather than mild cognitive impairment. The concept of vascular cognitive impairment has been introduced to emphasize the high prevalence of cognitive impairment when there is vascular damage to the brain. (
24) The term encompasses all degrees of severity of cognitive deficits resulting from vascular disease: some patients would not be considered to have dementia according to current definitions, others would meet the criteria for vascular dementia, and still others would be considered to have dementia, which would best be labelled as mixed Alzheimer disease and cerebrovascular disease. (
25–
27) The inclusion of the mildest form of cognitive impairment will enable patients and health care professionals to optimize preventive strategies before the stage of clear dementia is attained. In all of these individuals, there is good evidence that hypertension should be recognized and treated appropriately (see the section “Treatment of vascular factors”). The evidence for treatment of other risk factors is less compelling.
CONTROVERSIES IN MILD COGNITIVE IMPAIRMENT
Mild cognitive impairment is still a controversial clinical concept, with several different diagnostic criteria and definitions of memory loss being used. Depending on the diagnostic criteria used, the prevalence and incidence of mild cognitive impairment vary considerably. (
17,
28) Because of these uncertainties, some have opposed adoption of the term “mild cognitive impairment” for clinical use. (
29) However, the growing use of the term as a diagnostic entity has empowered physicians and patients and has given them a more accurate view of the nature of the patient's mild memory loss than was previously possible. (
30,
31) In addition, recognition of the condition at this stage may prove to be the optimum point at which to intervene with preventive therapies when they become available. (
32) Mild cognitive impairment will increasingly become a label used by neurologists, geriatricians and family physicians who have elderly patients with cognitive impairment.
One uncertainty is whether patients with mild depression should be excluded from consideration as having mild cognitive impairment. Some studies have shown that depression is present in up to 60% of patients with mild cognitive impairment who go on to have Alzheimer disease, and the presence of depression may in fact be a useful prognostic sign in patients with mild cognitive impairment. (
33)
Another challenge is the assessment of “significant impairment in functional ability,” which is one of the criteria for dementia, and hence important in separating mild cognitive impairment from dementia. Unfortunately, there is little agreement on the criteria for the evaluation of functional ability, and no agreement on what “significant” functional impairment entails. About 31% of patients with mild cognitive impairment probably have subtle functional changes, (
34) and one might even think the need to maintain written lists is a functional change. The key distinction for diagnosis of mild cognitive impairment is that the changes should affect only higher functions and not represent “significant impairment,” although this is crudely defined. The most sensitive questions for detecting functional impairment appear to be those that ask patients about whether they are maintaining their hobbies, are able to handle complex financial affairs, are able to use new equipment and tools, are finding themselves repeating questions, or are forgetting the month and year. (
35) There is also controversy about the best way to objectively measure memory loss, whether by means of brief cognitive testing or a full neuropsychological evaluation.
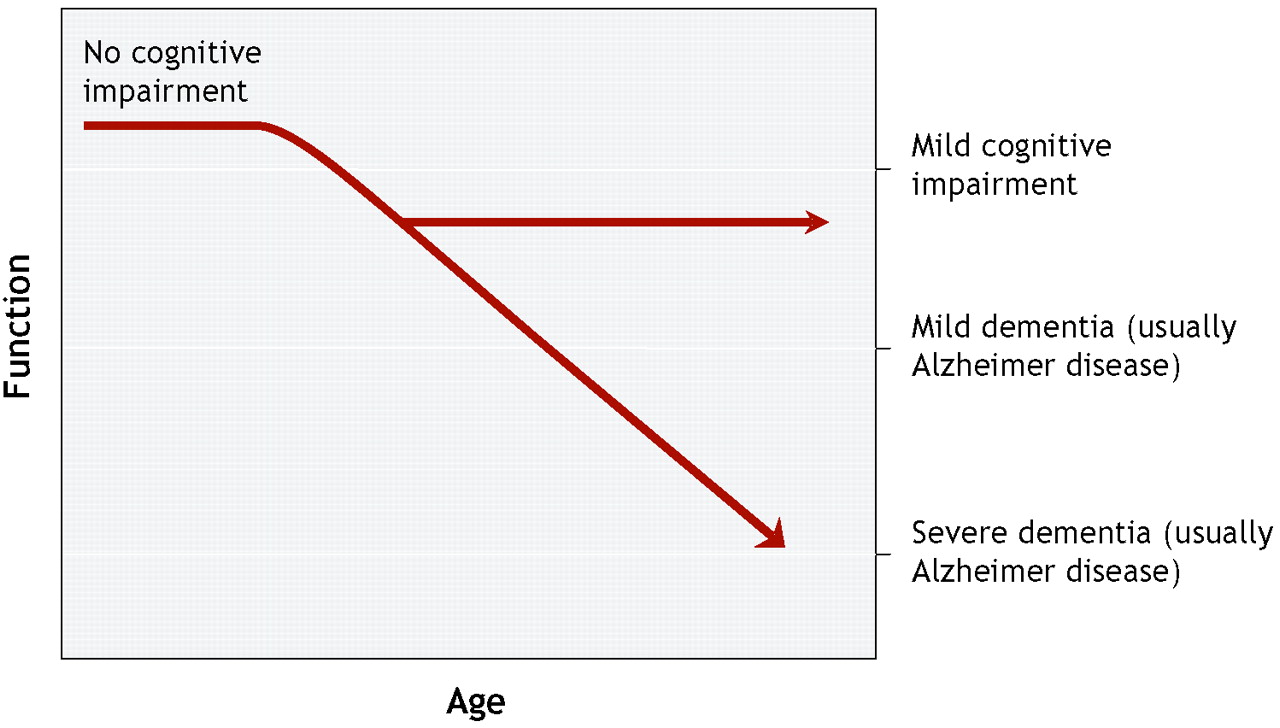
For all these reasons, it is usual to encounter subtle but significant variability in patients with mild cognitive impairment. (
36) Mild cognitive impairment is perhaps best viewed as a somewhat heterogeneous clinical syndrome that for some patients may be a prodrome for dementia (
Figure 1).
APPROACH TO DIAGNOSIS
Mild cognitive impairment should be detected and diagnosed because people with this condition are at increased risk for Alzheimer disease or other types of dementia compared with similarly aged individuals in the general population. (
22) There have been no studies that have validated a particular diagnostic approach. Some physicians suggest that the process for diagnosing mild cognitive impairment should be the same as that for diagnosing dementia, which includes history-taking and physical examination, brief cognitive testing, and laboratory tests to look for reversible causes of memory loss. (
10) Although neuroimaging with computed tomography or magnetic resonance imaging has been extensively investigated for its possible role in establishing a prognosis, we lack studies demonstrating that it is necessary for a family physician to carry out imaging in patients with mild cognitive impairment.
Until 2001, there were no specific brief cognitive tests to detect mild cognitive impairment. Although the Mini-Mental State Examination was considered useful, it had low sensitivity to detect mild cognitive impairment. (
37) Since that time, several brief instruments have been developed to help screen for mild cognitive impairment. Although they have not been compared in studies, 2 of the tests—the DemTect (
38) and the the Montréal Cognitive Assessment (
39)—have shown promising results in well-designed studies based on prespecified criteria for mild cognitive impairment. (
37)
The Montréal Cognitive Assessment, developed by some of the authors of this article, takes 10–15 minutes to administer and evaluates the following domains: delayed recall, verbal fluency, visuospatial skills, clock drawing, executive functions, calculation, abstraction, language, orientation, attention and concentration. In a validation study involving 277 French- and English-speaking participants recruited from a memory clinic, the test achieved a sensitivity of 90% and specificity of 87% to detect mild cognitive impairment in patients and distinguish them from normal controls. The positive predictive value was 89%, and the negative predictive value was 91%. The Montréal Cognitive Assessment and instructions can be obtained by physicians free of charge at
www.mocatest.org. The DemTect assesses word recall, number transcoding, semantic word fluency and digit span. In a validation study involving 363 English-speaking participants, the test achieved a sensitivity of 80% and specificity of 92% to detect mild cognitive impairment in patients and distinguish them from normal controls.
One important qualification of the Montréal Cognitive Assessment and DemTect is that neither has been fully validated in a family practice setting.
RISK OF PROGRESSION TO DEMENTIA
In the opening case of this article, Mr. W asks if he has early signs of Alzheimer disease and whether his condition will get worse. His concern is understandable, since patients classified as having mild cognitive impairment have a high rate of progression to dementia, particularly Alzheimer disease. Some others have pathological evidence of dementia with Lewy bodies or cerebral infarction. (
40) In most clinic-based studies of mild cognitive impairment, 40%–80% of patients who met the criteria for this condition were found to have Alzheimer disease during a 5-year follow-up, for an annual conversion rate of about 10%–15%. (
28,
41–
47) The conversion rate is substantially lower in population-based samples. (
48) What is not known is whether all people with mild cognitive impairment will go on to have dementia. In a 10-year follow-up study in a memory clinic, Visser and colleagues found the risk of progression to dementia to be 48% among 64 patients with mild cognitive impairment. (
49) In a Canadian study involving 89 patients with mild cognitive impairment recruited in a memory clinic, no disease progression occurred in about 25%, even 10 years after onset of memory problems. (
50–
52) The diagnostic category of mild cognitive impairment appears to contain many individuals who will go on to have Alzheimer disease or another type of dementia as well as a subgroup of people in whom no progression will occur and who may even be found to have no cognitive impairment at a second visit. (
42,
43,
45,
46) It is best to view people with mild cognitive impairment as a group at high-risk for dementia. (
43–
46,
53)
Is it possible to determine at initial presentation which people with mild cognitive impairment will go on to have Alzheimer disease in a limited period (e.g., 5 years)? There appear to be notable and measurable deficits in people in whom Alzheimer disease develops, a good number of years before the diagnosis is officially made on clinical grounds. (
54) Numerous attempts have been made to identify prognostic markers in mild cognitive impairment. Most involved rather small samples of patients followed for limited periods. (
55,
56) A number of biomarkers, imaging techniques and neuropsychological tests are under investigation (
Box 2), (
57–
82) with promising results of their predictive utility. However, insufficient evidence is available regarding their sensitivity, specificity, reproducibility and ease of use. Therefore, we cannot yet advocate their routine use for predicting progression to dementia in patients with mild cognitive impairment. Some combination of cognitive testing and imaging may, in the future, allow more accurate prediction. Until then, annual clinical follow-up is the best recommendation.
TREATMENT
The treatment of mild cognitive impairment has been the subject of a number of recent chapters and reviews. (
56,
83) There have been relatively few randomized controlled trials of any therapy sufficient to rank as level 1 evidence. Nevertheless, there are a number of potential interventions, both nonpharmacologic and pharmacologic, that deserve to be addressed.
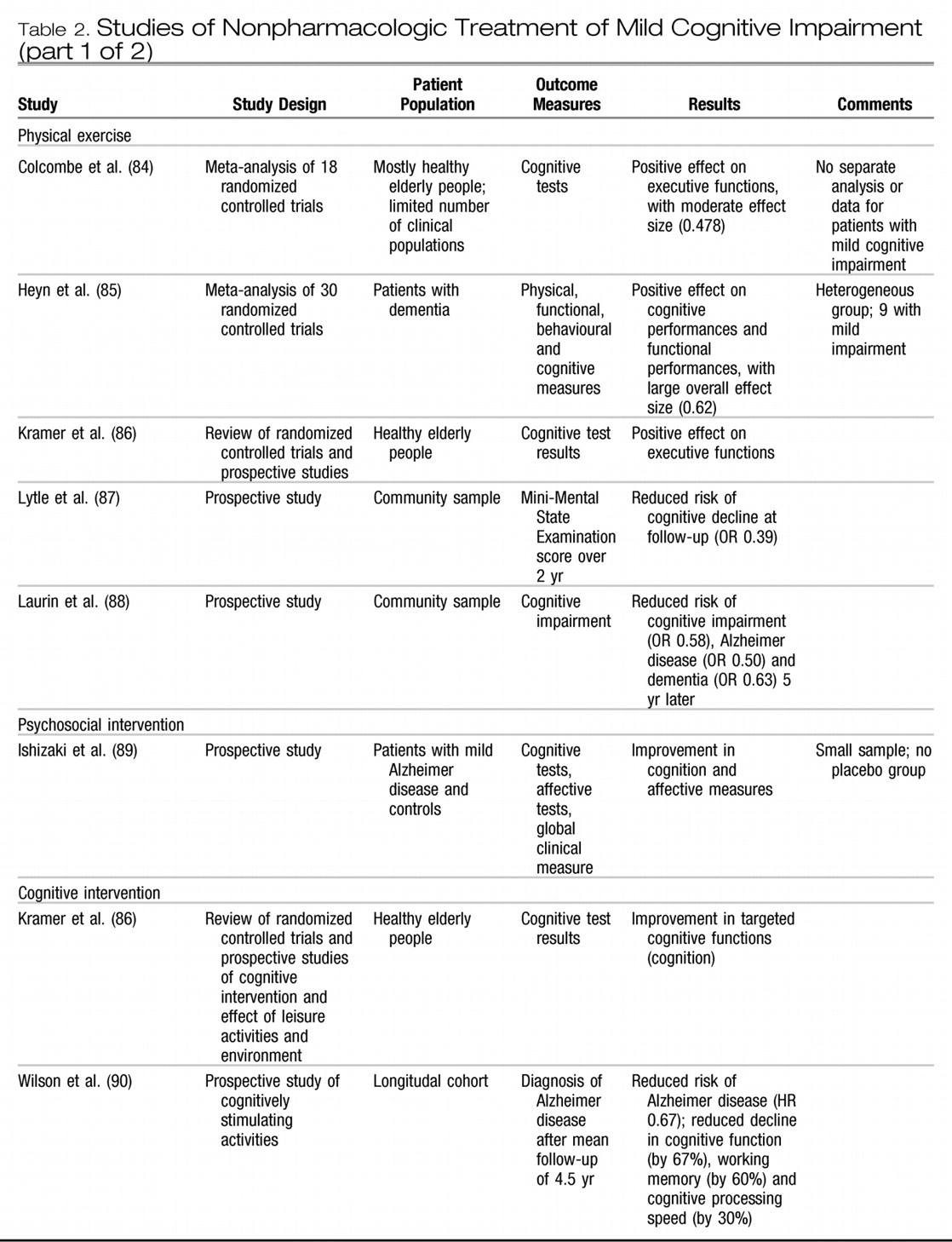
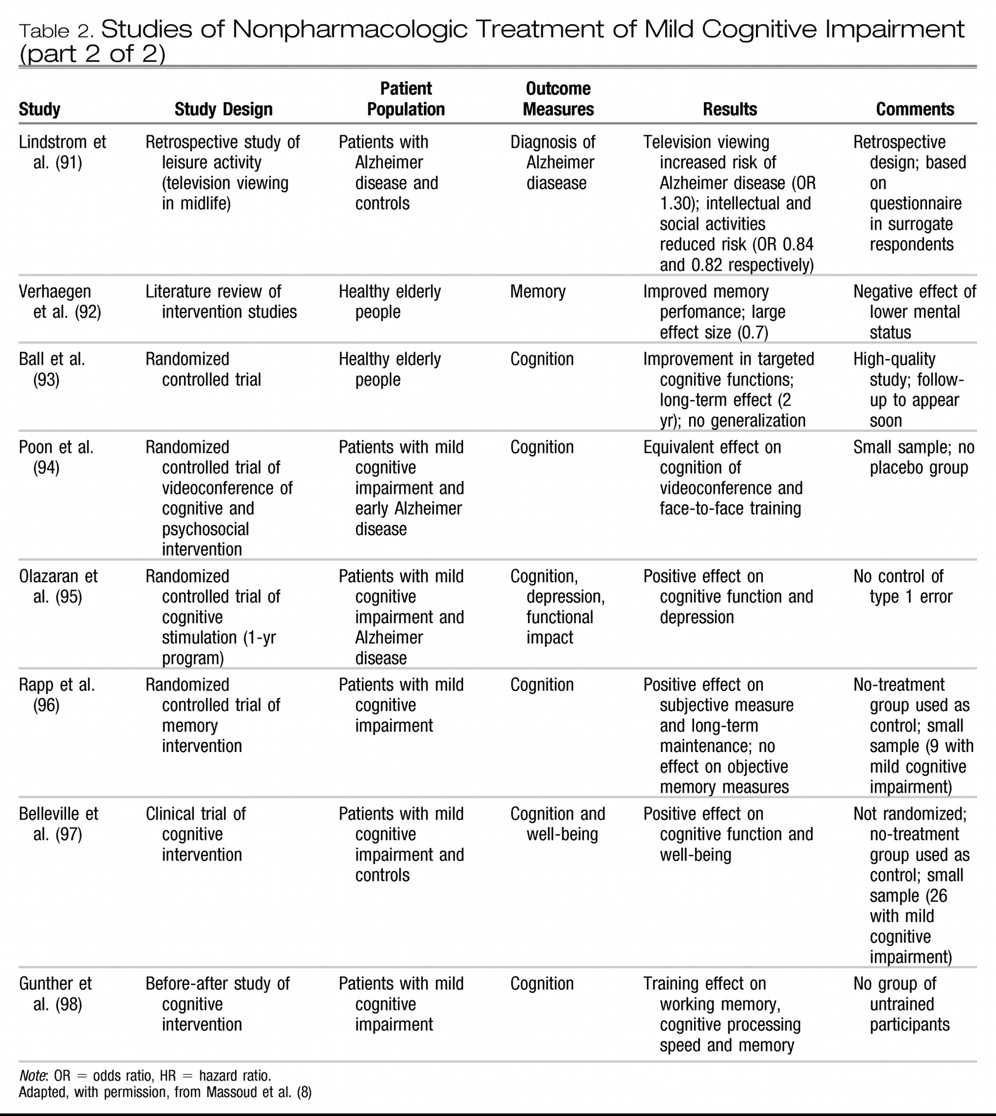
Table 2 provides additional information on studies relevant to nonpharmacologic treatment of mild cognitive impairment. (
84–
98)
NONPHARMACOLOGIC TREATMENT
Treatment of exacerbating and comorbid conditions There are a number of conditions that can exacerbate memory loss in mild cognitive impairment, or even produce mild cognitive impairment in an otherwise cognitively normal elderly individual. Attention to these factors is recommended, even in the absence of formal RCTs.
Patients with sleep disorders often present with memory loss, (
99) and this seems a reasonable factor to assess and treat. (
99–
101) Referral for assessment of sleep apnea in a specialty sleep clinic is recommended if the patient reports a sleep problem.
The overlap between dementia and depression continues to be an important area, and untreated depression will exacerbate and amplify memory loss. (
102–
106) If present, depression in mild cognitive impairment and early Alzheimer disease should be treated with appropriate nonpharmacologic and pharmacologic approaches.
Isolation has also been proposed as an exacerbating factor. An important study in the Kungsholmen district of Stockholm (
107) demonstrated that a poor or limited social network increased the risk of dementia by 60%, and a significant gradient was found for increasing degrees of social connections. An extensive social network appears to protect against dementia. Clearly this requires a lifelong commitment to building social interactions, but this may be a modifiable risk, and patients with mild cognitive impairment should be encouraged to have social interactions. Nevertheless, the literature on all of these factors is meager, and no specific recommendations can be made on any of these items.
COGNITIVE INTERVENTIONS
Longitudinal cohort studies involving healthy elderly people have shown that engagement in stimulating cognitive activities (engaged lifestyle; novel and intellectually challenging activities) is associated with better memory and verbal abilities compared with people not engaging as much in such activities. (
86) Participation in intellectually stimulating and social activities in midlife has been associated with reduced risk of Alzheimer disease. (
90,
91) One large-scale randomized controlled trial of cognitive interventions (training memory, cognitive processing speed or reasoning v. no training) was completed in a sample of 2832 healthy older adults. (
93) The results indicated improved performance following training in the cognitive domains that were targeted by the interventions. The positive effects (less decline in cognitive function and in complex daily activities) were sustained over a 2-year and even a 5-year follow-up, and the effect sizes were moderate to large. (
108) There is good evidence that cognitive training increases cognitive efficacy on target measures in healthy older adults.
Two nonrandomized studies and 2 randomized controlled trials, all with relatively small samples, reported that cognitive training was associated with long-term improvement in cognitive performance (working memory and verbal episodic memory) in people with mild cognitive impairment. (
95,
96,
98) Belleville and colleagues, (
97) in a study of a multifactorial program for memory training versus no training involving 28 participants with mild cognitive impairment, reported larger memory improvement in the trained group than in the group without training. Moderate to large effect sizes were observed for the training effect on target episodic memory measures.
Studies investigating the effect of cognitive interventions in mild cognitive impairment have provided encouraging findings. However, the effort required to implement such therapeutic measures is not trivial, and large-scale cognitive interventions would require considerable resources. Before this therapy can be recommended for widespread use, more research is required in the form of properly designed randomized controlled trials with larger samples. The evidence at present is insufficient to conclude that organized cognitive intervention is beneficial in preventing progression in mild cognitive impairment. In terms of general intellectual activity, we have alluded to population-based studies only, showing that participation in intellectually stimulating and social activities in midlife has been associated with reduced risk of Alzheimer disease. (
90,
91) These data do not provide the same level of evidence as would come from randomized controlled trials of the effect of beginning such activities at age 65, or when mild cognitive impairment is diagnosed, to prevent or delay progression to Alzheimer disease compared with controls. On the other hand, given that there is little or no harm associated with general engagement in stimulating cognitive activities, physicians and other health professionals can promote engagement in cognitive activities as part of an overall healthy lifestyle for elderly patients with and without memory loss (
Box 1). The data to date suggest that cognitive intervention is more efficient in people with relatively mild cognitive deficits (healthy older adults and people with mild cognitive impairment) than in people with mild dementia. Cognitive intervention is also more efficient when provided in structured programs provided to small groups, which allows the therapist to adapt the approach to the individual needs and capacities of the participants.
PHYSICAL ACTIVITY
Several longitudinal cohort studies involving elderly people without cognitive impairment at enrolment have indicated that physical exercise is associated with reduced cognitive decline and reduced risk of dementia. (
87,
88) However, other studies have failed to show a protective effect of physical exercise on cognitive decline and dementia. (
109) Two recent meta-analyses of the impact of physical exercise programs on the cognitive function of older adults reported moderate effect sizes for exercise training on global cognitive scores, (
84,
85) with a larger effect on tasks measuring executive control reported by Colcombe and Kramer. (
84) There are immense implications of such research in terms of potential public health measures to prevent dementia and cognitive decline. More randomized controlled trials are needed to assess the optimal forms of exercise training in older adults, particularly in terms of intensity and duration. Safety issues will also need to be better addressed. Finally, no studies have been carried out involving specifically people with mild cognitive impairment to assess the effect of physical training on their cognitive capacities and cognitive decline. Nevertheless, it is recommended that physicians and other health professionals may promote physical activity, at an intensity level that is adapted to the person's overall physical capacities, as part of a healthy lifestyle for older individuals with and without memory loss.
TREATMENT OF VASCULAR RISK FACTORS
Several epidemiologic studies have shown an association between vascular risk factors and cognitive impairment. (
110–
113) Results from 3 clinical trials (
114–
116) have supported the role of treatment of hypertension in reducing the risk of cognitive decline in elderly people without dementia, some of whom had mild cognitive impairment. The Syst-Europe randomized controlled trial (
114) involved 2418 older people with isolated systolic hypertension and showed a 50% decrease in cases of dementia after 2 years in the treatment group compared with the control group. By extrapolation of these results, if 1000 hypertensive patients were treated with antihypertensive drugs for 5 years, 19 cases of dementia might be prevented. Another study of blood pressure reduction in elderly people with cerebrovascular disease resulted in significant but less impressive reduction in cognitive decline and dementia. (
115) The third clinical trial compared captopril and bendrofluazide in people with mild cognitive deficits. (
116) Patients with the best response to treatment in terms of reduction of their diastolic blood pressure showed significant improvement on 2 cognitive tests.
Findings from these 3 studies suggest that the reduction of blood pressure in elderly patients with hypertension who have mild cognitive impairment is not hazardous. The evidence that treating other vascular risk factors will reduce subsequent dementia is meager. Trials of primary prevention with statins, for instance, did not provide any evidence of benefit on cognitive function. (
117,
118) We recommend treatment of vascular risk factors, including hypertension, as an effective means of preventing progression to dementia in patients with mild cognitive impairment (
Box 1).
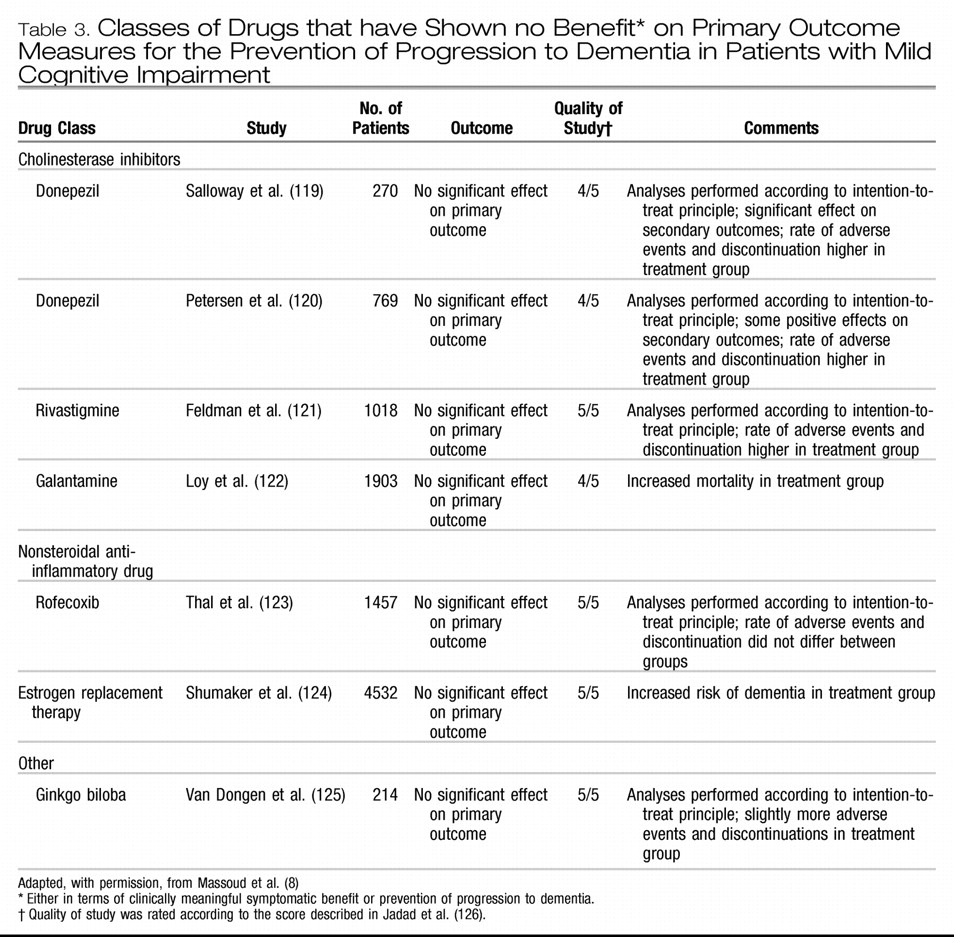
PHARMACOLOGIC TREATMENT
Several classes of drugs have been studied for the prevention of progression to dementia. These include cholinesterase inhibitors, nonsteroidal anti-inflammatory drugs, estrogen replacement therapy, ginkgo biloba and vitamin E. The studies have shown no benefit either in terms of clinically meaningful symptomatic benefit or prevention of progression to dementia in patients with mild cognitive impairment (
Table 3). (
119–
125) In a study of the cholinesterase inhibitor galantamine, mortality was increased in the treatment group compared with the control group. (
122) Details on the literature are available in reviews (
55,
56,
83) and the background papers for this article. (
7,
8) Given the findings of these studies, we do not recommend the use of these medications for the treatment of mild cognitive impairment (
Box 1).
Other classes of drugs currently being studied include statins, cholesterol-lowering drugs, anti-amyloid drugs (e.g., β-secretase inhibitors, gamma-secretase inhibitors, glycosaminoglycan mimetics, amyloid immunotherapy) and ampakines.
KNOWLEDGE GAPS
In this article, we have alluded to several gaps in our knowledge. The most fundamental of these is whether establishing “mild cognitive impairment” as a clinical diagnosis will improve our care of elderly patients and lead to better strategies for preventing dementia and whether it will increase a patient's anxiety. There are no studies to date that critically examine the psychological or social effects of using the label “mild cognitive impairment” in a clinical context. It is our clinical impression, however, that there are psychological benefits to a patient in being given a diagnosis of mild cognitive impairment, as opposed to being told (erroneously) that he or she is “normal” or that “the doctor doesn't know what it is.”
We lack easy, reliable and useful tools for predicting which people with mild cognitive impairment will go on to have dementia. Large observational studies are required, and some are underway, to understand the complex factors involved in the evolution of mild cognitive impairment and the interplay of risk factors in the development to dementia. Paradoxically, mild cognitive impairment is a term that may be used less, or not at all, once reliable, inexpensive biomarker or physiologic tests become available. (
127)
If mild cognitive impairment is to remain with us as a diagnosis, one limitation is the lack of standardization of how to apply this diagnosis in practice. For instance, there is little agreement on how to define “largely preserved function,” or how physicians should assess function. For that matter, “significant impairment in function” (a basic component of the definition of dementia) remains poorly defined.
There is a need for longitudinal studies to validate any diagnostic approach. Currently, no accepted or recommended way to diagnose or screen for mild cognitive impairment exists. We lack studies, for instance, that demonstrate whether structural imaging will have an impact on the ultimate outcome of patients with mild cognitive impairment.
Larger trials are needed to determine the efficacy of all of the pharmacologic and nonpharmacologic interventions being considered. For instance, it has been shown that a poor or limited social network increases the risk of dementia substantially, but we do not know whether programs to increase the social interactions of elderly people would protect against the development of dementia.
THE CASE REVISITED
In the case described at the beginning of this review, Mr. W would meet the criteria for mild cognitive impairment. This is because there are subjective complaints of memory loss from both Mr. W and his family, and he has objective short-term memory loss with preserved daily function. Mr. W was administered the Mini-Mental State Examination as a brief cognitive test to confirm the objective memory loss. Brief cognitive testing with the Montréal Cognitive Assessment or DemTect might reveal even further clear evidence of cognitive impairment. If full neuropsychological testing is available, it would also serve to better document objective memory impairment, assess which other cognitive domains are involved and provide a baseline for the future. Such neuropsychological testing is by no means obligatory, and most family physicians would be content to follow the patient's progress with annual re-examination.
A patient with mild cognitive impairment such as Mr. W should probably have the same set of blood work as would be ordered for a patient with dementia, although there is currently no literature that speaks to this question in mild cognitive impairment. Mr. W's diagnostic workup would include the laboratory tests currently being used for patient with early dementia (complete blood count, and measurement of thyroid stimulating hormone, vitamin B12, serum calcium and electrolytes). Mr. W has hypertension as a vascular risk factor, but he has never had a transient ischemic attack or stroke. It would be appropriate in this case to perform structural imaging with computed tomography to evaluate possible contributing cerebrovascular disease. Even if the scan shows some silent cerebral infarcts, Mr. W's diagnosis of mild cognitive impairment would not change, but stronger motivation would exist to aggressively control his vascular risk factors.
Mr. W should be counselled that he has mild cognitive impairment and is therefore at high risk for progression to dementia over the coming years (from half to three-quarters of such patients seen in memory clinics go on to have dementia over 10 years). It is not advisable to falsely reassure him that he is normal. At the same time, there exists a real chance that his condition will not progress to dementia, and there is thus some room for optimism. The prognosis is not clear, and there are no current widely available means of establishing a prognosis. Therefore, annual clinical reassessment should be recommended.
Treatment strategies should be discussed. Exercise and engagement in stimulating cognitive activities should be encouraged, both with the hope of preserving memory and as part of a healthy lifestyle for a person with vascular risk factors. The importance of sleep and managing stress should be reviewed, and Mr. W should be advised to stop the use of diazepam at night. He should be encouraged to continue with rigorous blood pressure control as an important element of memory preservation over the coming years. No other specific pharmacologic therapy should be undertaken at this juncture.
CONCLUSION
Physicians will increasingly see elderly patients with mild memory loss, and learning an approach to diagnosing states such as mild cognitive impairment is now warranted. Family physicians should be familiar with the criteria for mild cognitive impairment and start to use them in practice. A review of the literature provides evidence that leisure activities, cognitive stimulation and physical activity should be promoted as part of a healthy lifestyle in elderly people and those with mild cognitive impairment. Vascular risk factors should be treated optimally in these people as well. No other specific therapies can yet be recommended, or supported as having adequate demonstration of efficacy.
It is our hope that, in the future, asymptomatic people will have their Alzheimer disease detected and diagnosed long before symptoms appear, much as we currently screen for diabetes by measuring serum glucose measurements, long before diabetic symptoms occur. In this future scenario, the utility of a clinically heterogeneous label such as mild cognitive impairment may well disappear. In the meantime, this clinical label aids physicians and empowers patients and has a place in the general physician's clinical knowledge base.