Patient Management Exercise for Genetics and Genomics
Abstract
CASE VIGNETTE PART 1
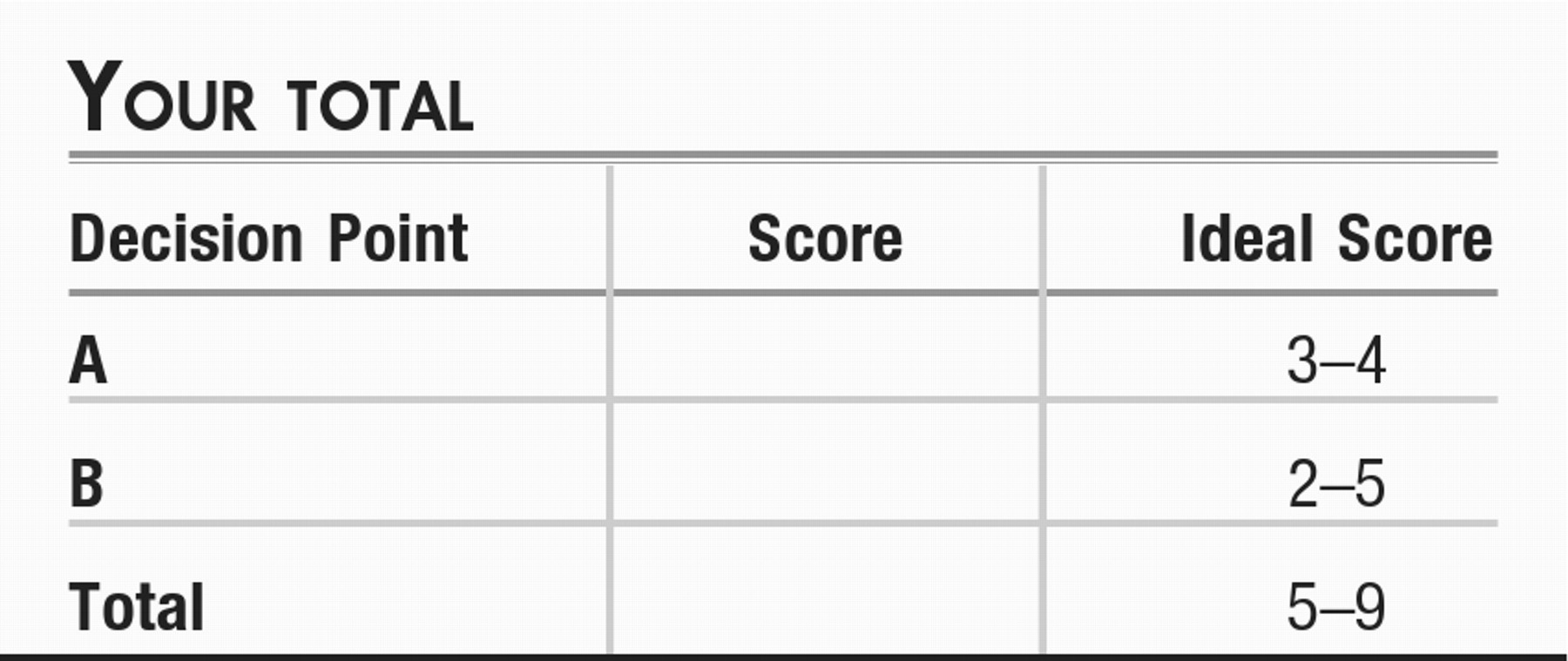
Decision Point A:
| A1._____ | Bipolar disorder instead of major depression as the diagnosis. |
| A2._____ | Inadequate therapeutic trials. |
| A3._____ | Covert substance use. |
| A4._____ | Secondary gain. |
CASE VIGNETTE PART 2
Decision Point B:
| B.1._____ | Offer a trial of augmenting the venlafaxine (still at 37.5 mg/day) with mirtazapine (starting with 15 mg/day). |
| B.2._____ | Draw a blood sample for genotyping. |
| B.3._____ | Refer her for psychotherapy. |
ANSWERS: SCORING, RELATIVE WEIGHTS, AND COMMENTS
Decision Point A:
| A1. | Depression as a part of bipolar disorder. (+1) Nonresponse to treatment can raise the question that the diagnosis is in error. Although clinical lore suggests that feeling a boost of energy with an selective serotonin reuptake inhibitor may increase one's suspicion that a person has bipolar disorder, this side effect is nonspecific and can be found in individuals with unipolar major depressive disorder as well. Had there been the presence of a family history of mood disorder, one's suspicion might have been somewhat sharpened, depending on the nature of the disorder in a relative, but the absence of a family history does not help with this differentiation. Common things happen commonly, and the prevalence of unipolar major depressive disorder is far greater than that of bipolar disorder. |
| A2. | Inadequate therapeutic trials. (+3) In STAR*D, it was found that half of the individuals who eventually had a remission with citalopram did so in 6 weeks or less, but that the other half took up to 12 weeks to cross the “finish line” of achieving remission (2). The Antidepressant Treatment History Form (3) is a common research tool used to assess the adequacy of past treatment trials. Intolerable side effect problems often can lead to abandoning a particular medication; here, the patient was able to persist with her maximal tolerated doses for many weeks, but dosing was limited by tolerability. |
| A3. | Covert substance use. (0) Many substances of abuse can produce depressive symptoms either during intoxication (e.g., sedative and hypnotic agents) or during withdrawal (e.g., psychostimulants). Although individuals in their 20s may be an at-risk age group for substance use, this patient's history and presentation allow you to put this far lower on the differential. |
| A4. | Secondary gain. (−1) As psychiatrists, we rely on self-report of our patients for data to make diagnoses and assess response to treatment. We also must rely on patients to be independently adherent to treatment when daily ingestion of a medication is concerned. A tactful approach is needed when one is probing patterns of adherence or psychosocial factors that might lead to persistence of symptoms to avoid disrupting the trust needed in a therapeutic relationship, whether the primary intervention is psychotherapeutic or pharmacological. We have no evidence that factors such as disability insurance, avoiding obligations by adopting the “sick role,” or other considerations are at play here. |
Decision Point B:
| B1. | Augmentation treatment. (+1) The patient has had trouble tolerating dose titration for two antidepressants but is able to take a low dose of venlafaxine. Based on findings in STAR*D (3) and other studies (4), you consider augmenting with mirtazapine, with thoughts to capitalize on their complementary putative mechanisms of action. You also hope that the effect profile of mirtazapine will help with her insomnia and weight loss. |
| B2. | Draw blood for genotyping. (+2) The history of medication intolerance for some medications and lack of efficacy for codeine makes you suspicious that the patient may carry genes that confer atypical drug metabolism. |
| B3. | Psychotherapy. (+2) Studies of cognitive behavior therapy have shown that this modality may have efficacy comparable to that of pharmacotherapy for some patients (5). Although physical side effects are not generally an issue with cognitive behavioral therapy, some individuals may find concerns that limit their ability to adhere to a treatment course, such as time demands, insurance coverage, and openness to a psychotherapeutic approach. |
CONCLUSIONS
REFERENCES
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBGet Access
Login options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

