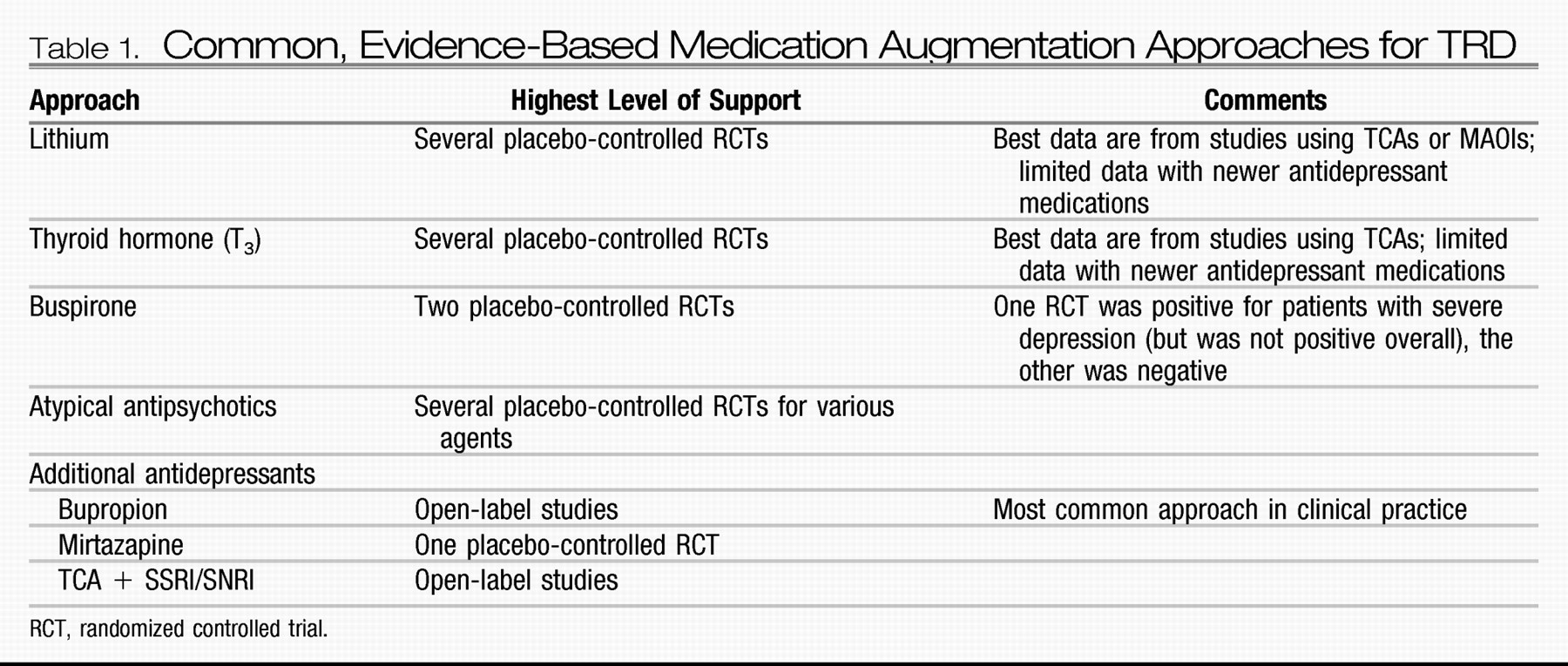
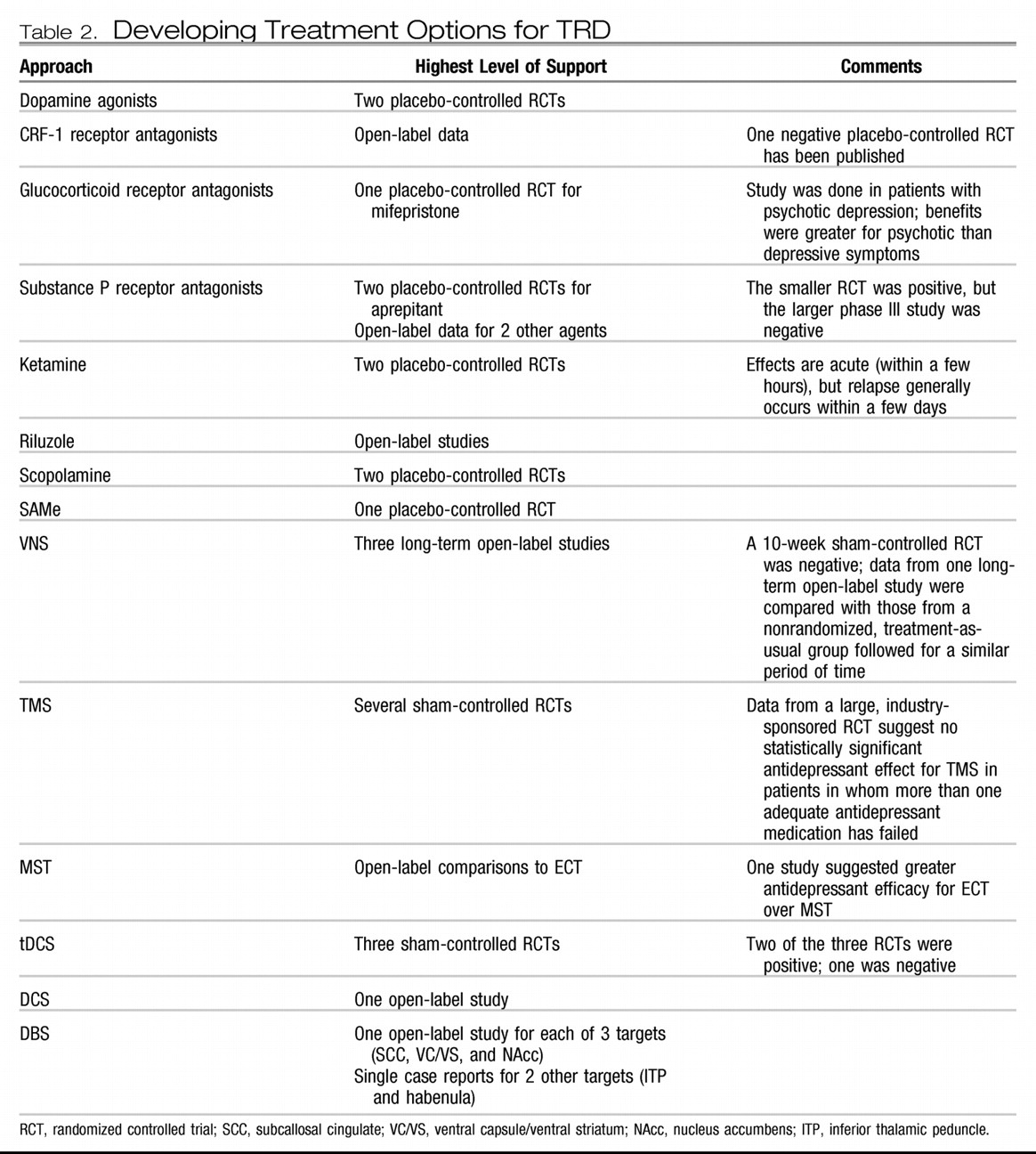
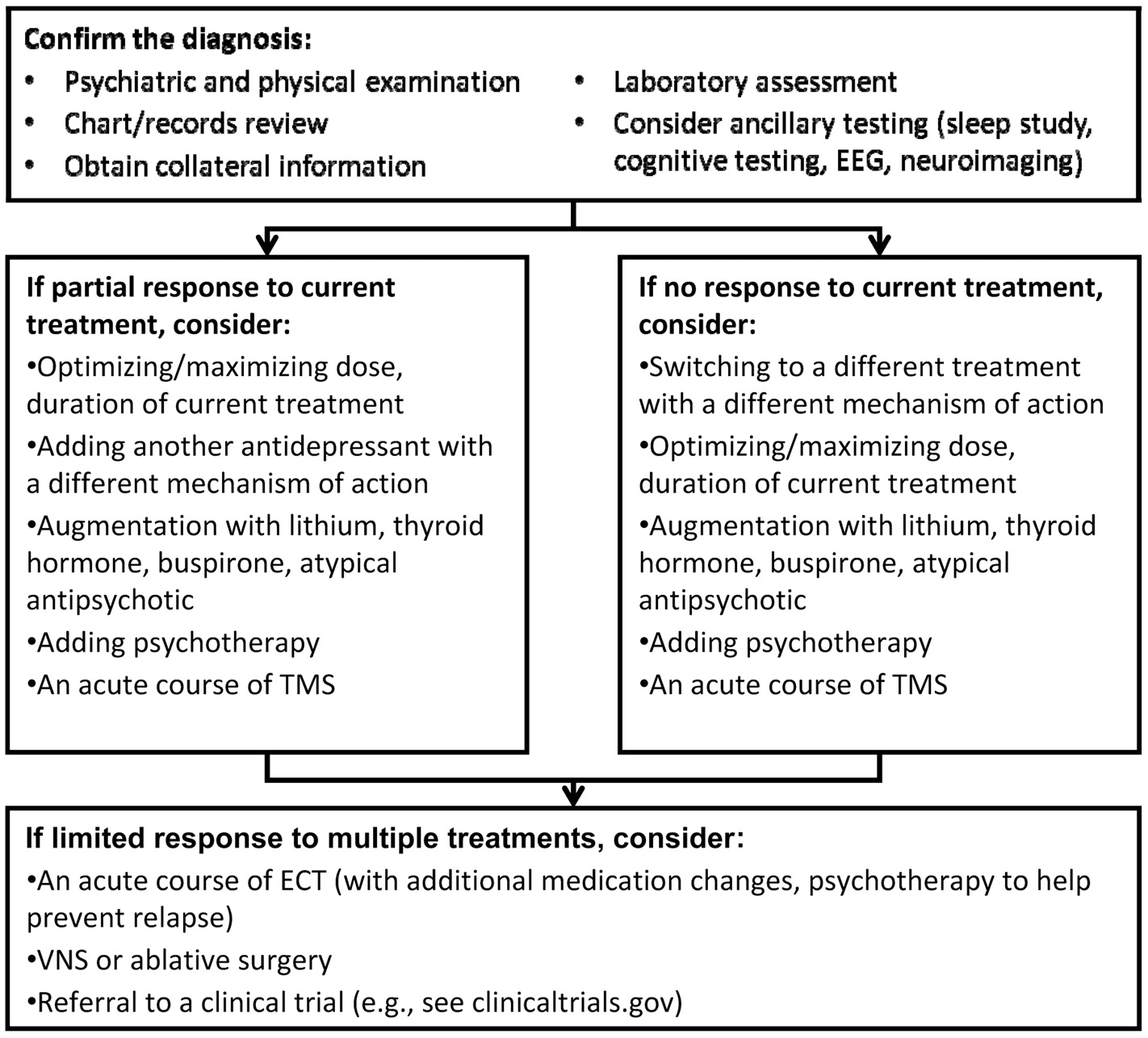
The emergence of focal brain stimulation therapies over the past few decades has been jointly facilitated by major advances in neuroimaging and the technical ability to acutely and chronically stimulate a discrete neural target. Neuroimaging studies have helped map out a network of brain regions involved in the pathophysiology of depression and the neurobiological mechanisms of various treatments (
128,
129); this work has helped postulate critical nodes within this network that might reasonably serve as targets for direct modulation. Such focal neuromodulation is now possible via noninvasive acute stimulation techniques [e.g., transcranial magnetic stimulation (TMS) and transcranial DC stimulation (tDCS)] as well as methods that allow for chronic stimulation but require surgery [e.g., vagus nerve stimulation (VNS), direct cortical stimulation (DCS), and deep brain stimulation (DBS)]. Two of these procedures (VNS and TMS) are currently approved by the FDA for the treatment of depression.
VNS.
VNS involves stimulating the vagus nerve via an electrode that is surgically attached to the nerve where it courses through the neck. A subcutaneously implanted pulse generator (IPG) controls stimulation and serves as the power supply for the system. Common treatment parameters include chronic but intermittent stimulation (e.g., 30 seconds on every 5 minutes). In 1997, VNS was approved by the FDA for the treatment of medication-resistant epilepsy. Observations of positive mood effects in some patients with epilepsy led to testing in medication-resistant depression. A double-blind, sham-controlled trial (on versus off stimulation) showed no statistically significant antidepressant effects for 10 weeks of active VNS, with a response of 15% for active VNS versus a 10% response rate with sham VNS (
130). However, open-label data suggest increasing antidepressant efficacy over a year of stimulation [in combination with treatment-as-usual (TAU)] in patients in whom between two and six adequate treatments in the current episode have failed, with reported response rates of 27%–53% and remission rates of 16%–33% (
131–
133). Response and remission rates appear to either remain stable or may continue to increase with 2 years of stimulation (
134,
135).
In a nonrandomized comparison with patients with TRD receiving only TAU, 1-year response rates were statistically significantly higher in the VNS + TAU group (27% versus 13%). In addition, VNS + TAU only showed a 23%–35% relapse rate over an additional year of stimulation (
136) compared with a 62% relapse rate in the TAU-only group over an equivalent time period (
137). However, a European study suggested that only 44% of patients receiving VNS sustain a response over an additional year of stimulation (
133).
Risks of VNS surgery are minor, and adverse effects associated with acute and chronic stimulation include voice changes, coughing, and difficulty swallowing. In general, VNS appears to be cognitively safe, although stimulation intensity may be associated with some modest cognitive impairments (
80,
138). The published data suggest that more than 80% of patients receiving VNS choose to continue stimulation even in the absence of an antidepressant response, suggesting that the treatment is generally well-tolerated.
TMS.
TMS uses an electromagnetic coil placed on the head to generate a depolarizing electrical current in the underlying cortex. Repetitive TMS (rTMS) delivers a train of stimuli at a set frequency, with “high-frequency” denoting ≥5 Hz stimulation and “low-frequency” denoting ≤1 Hz stimulation. A series of stimulation trains are given during each treatment session, and a typical treatment course involves daily sessions (each lasting about 1 hour) for 3–6 weeks. Patients are awake during treatment, and no anesthesia is required.
The types of rTMS most commonly studied for the treatment of depression include high-frequency rTMS (generally 5–20 Hz) applied to the left dorsolateral prefrontal cortex (DLPFC) and low-frequency rTMS (generally 1 Hz) applied to the right DLPFC. Safety and efficacy have been demonstrated for both approaches through a number of relatively small open-label and sham-controlled studies (
139–
144). Although effect sizes in favor of active rTMS have been moderately strong, absolute response rates have been relatively low (generally between 20% and 40% in sham-controlled studies).
Higher stimulation intensity and total number of pulses delivered (i.e., longer treatment sessions and more sessions over time) are associated with better antidepressant effects (
145). High-frequency rTMS seems to be less effective in psychotic versus nonpsychotic depressed patients (
67) and those with a longer versus shorter (<5 years) duration of the current episode (
146). One study suggests lower efficacy in patients with late-life depression (
147), although it is noted that this study probably did not use optimal treatment parameters, and the efficacy of rTMS may be lower if stimulation intensity is not adjusted upward to account for prefrontal cortical atrophy (
148,
149), which was not done in this study of older patients.
Two multicenter, randomized, sham-controlled studies have helped clarify the safety and efficacy of rTMS as a monotherapy for medication-resistant depression. In an industry-sponsored study of medication-free patients who had not responded to at least one antidepressant medication, 4–6 weeks of high-frequency left DLPFC rTMS was associated with statistically significant antidepressant effects compared to sham rTMS, including higher response (24% versus 12%) and remission rates (14% versus 6%) after 6 weeks of treatment (
150). However, a secondary analysis showed that the difference in antidepressant efficacy between active and sham rTMS was only statistically significant in those patients in whom no more than one medication (of adequate dose and duration) had failed in the current episode (
151). A four-site National Institute of Mental Health-funded study using stimulation parameters and eligibility criteria similar to the industry-sponsored study also showed statistically significant antidepressant effects for active rTMS with remission rates similar to those in the industry study (
152). These data also suggested that rTMS was more effective in patients with a lower degree of medication resistance. The long-term efficacy of rTMS is largely unknown, with studies suggesting relapse rates similar to those seen after ECT (
153,
154). Repeated rTMS courses may be beneficial in helping to maintain benefit over time (
155).
Although rTMS can result in seizures, this is highly unlikely when stimulation parameters are maintained within suggested safety guidelines (
156). Common side effects of rTMS include pain at the site of stimulation and headaches, although most patients tolerate treatments very well (
150,
152). There are no cognitive side effects associated with rTMS for depression (
80). A potential disadvantage of rTMS as currently administered is the need for daily treatments over several weeks. However, in a recent open-label study testing accelerated rTMS (in which 15 treatment sessions were delivered over 2 days in an inpatient setting) (
157), response and remission rates immediately after treatment were 43% and 29%, respectively, and were largely maintained over the next 6 weeks; side effects were similar to and no more severe than those seen with daily rTMS.
DBS.
DBS is achieved by placing a thin electrode through a burr hole in the skull into a specific brain region using imaging-guided stereotactic neurosurgical techniques. Electrodes can be placed in essentially any brain region and can be implanted unilaterally or bilaterally. Each electrode typically contains several distinct contacts that can be used to provide monopolar or bipolar stimulation. As with VNS and DCS, the electrodes are connected via subcutaneous wires to an IPG that controls stimulation.
DBS is an established treatment for patients with severe, treatment-resistant Parkinson's disease, essential tremor, and dystonia. DBS has largely replaced ablative surgery in these conditions because it can be adjusted to achieve maximal benefit with a minimum of side effects and can be turned off or completely removed in the case of severe, unwanted side effects. DBS of the anterior internal capsule [as a potential replacement for capsulotomy; this target is also referred to as the ventral capsule/ventral striatum (VC/VS)] has shown potential safety and efficacy for patients with severe treatment-refractory OCD based on a multicenter open-label case series of 26 patients (
167). This intervention is now FDA-approved via a Humanitarian Device Exemption. DBS of the subthalamic nucleus has also shown efficacy for OCD in a 6-month sham-controlled study (
168).
Based on a converging neuroanatomical database suggesting a critical role for Brodmann area 25 in a neural network involved in depression and antidepressant response, Mayberg and colleagues (
169,
170) demonstrated that open-label subcallosal cingulate DBS was associated with antidepressant effects in a cohort of 20 patients with severe TRD in whom at least four treatments had failed in the current episode. After 6 months of chronic stimulation, 60% of patients achieved response and 35% achieved remission; these effects were largely maintained over an additional 6 months with 72% of 6-month responders still meeting the response criteria at 12 months (and three additional patients achieving response by 12 months). Adverse events related to the procedure included skin infection (leading to explantation in two patients) and perioperative pain/headache related to surgery. There were no negative effects associated with acute or chronic DBS, and no negative cognitive effects (
171).
In studies of VC/VS DBS for OCD, it was noted that many patients experienced significant improvement in comorbid depression (
167), leading to testing of VC/VS DBS for TRD without comorbid OCD. After 6 months of chronic stimulation in 15 patients with TRD enrolled in a three-center open-label pilot study, 40% of patients achieved response and 27% achieved remission (
172). At the last follow-up (an average of 24 ± 15 months after onset of stimulation, with a range of 6–51 months), there was a 53% response rate and a 33% remission rate. Adverse effects related to surgery and/or the device included perioperative pain and a DBS electrode break. Adverse effects of stimulation included hypomania, anxiety, perseverative speech, autonomic symptoms, and involuntary facial movements; these were mostly reversible with a stimulation parameter change. No cognitive side effects were described.
The most ventral aspect of the VC/VS DBS target includes the nucleus accumbens, a region implicated in reward processing. This region was targeted for DBS in a cohort of 10 patients with TRD in an open-label pilot study, with 50% of patients achieving an antidepressant response after 6 months of chronic stimulation (
173). Case reports have described potential antidepressant efficacy of DBS of the inferior thalamic peduncle (which contains thalamocortical projection fibers) (
174) and the habenula (which is involved in modulation of monoaminergic neurotransmission) (
175).