Obsessive-compulsive disorder (OCD) is a severe and disabling illness (
1). Serotonin reuptake inhibitors (SRIs) (e.g., clomipramine and various selective serotonin reuptake inhibitors [SSRIs]) and cognitive-behavioral therapy (CBT) involving exposure and ritual prevention have both been found to be efficacious in randomized, controlled trials (
2). In clinical practice, SRIs are used most frequently (3), but because they typically yield only a 20%–40% reduction in OCD symptoms (
4), many SRI responders continue to have clinically significant symptoms.
The only SRI augmentation strategy with proven efficacy in multiple randomized, placebo-controlled trials involves the addition of antipsychotics (e.g., haloperidol, risperidone, olanzapine, or quetiapine) (
5). However, at most only half of the patients respond (i.e., experience ≥25% reduction in OCD severity) (
6,
7) and antipsychotics can cause significant adverse effects (
8).
Because of the efficacy of exposure and ritual prevention as monotherapy for OCD (
9) and promising findings from our open SRI augmentation trial using exposure and ritual prevention (
10), we conducted a randomized, controlled trial to compare the effects of augmenting SRIs with exposure and ritual prevention versus stress management training, another form of CBT. Stress management training teaches anxiety management skills (e.g., relaxation or problem solving) previously found ineffective for reducing OCD symptoms in adults (
11,
12). We used stress management training to control for attention, time, homework effort, and other nonspecific psychotherapy effects.
Additional data, published since the trial's initiation in 2000, indicate that exposure and ritual prevention can augment SRIs in the treatment of OCD (
13–
16). In the only prior randomized, controlled trial, Tenneij et al. (
17) compared the effects of just continuing medication (paroxetine or venlafaxine) versus adding exposure and ritual prevention. Adding exposure and ritual prevention (eighteen 45-minute sessions over 6 months) was superior, but the effects were modest. However, the study included patients with mild OCD (based on a baseline Yale-Brown Obsessive Compulsive Scale mean score=14, SD=6), lacked a psychotherapy control condition, and excluded patients with comorbid depression.
In comparison, in the present study, we recruited patients with OCD of at least moderate severity despite an adequate SRI trial (based on a Yale-Brown Obsessive Compulsive Scale score ≥16), compared the addition of exposure and ritual prevention with the addition of stress management training (a credible psychosocial control condition), used a twice-weekly therapy format proven efficacious in prior trials of exposure and ritual prevention (
10,
18), and included patients with comorbid depressive and anxiety disorders if OCD was the principal diagnosis. On the basis of the literature (
10–
12), we hypothesized that augmentation with exposure and ritual prevention would be superior to augmentation with stress management training in reducing OCD symptoms and improving functioning and quality of life in OCD patients who remain symptomatic despite an adequate SRI trial.
METHOD
Study design
This study was conducted at two academic outpatient clinics: the Anxiety Disorders Clinic, New York, and the Center for the Treatment and Study of Anxiety, Philadelphia. Patients were recruited between November 2000 and November 2005 by advertisements, word of mouth, and clinical referral. Each site's institutional review board approved the study. Patients provided written informed consent.
Participants
Eligible participants were between the ages of 18 and 70, had a DSM-IV diagnosis of OCD for at least 1 year as their principal psychiatric diagnosis, and reported at least minimal improvement from an adequate SRI trial while remaining at least moderately ill (based on a Yale-Brown Obsessive Compulsive Scale score ≥16). From the literature (
4,
19–
23), an adequate SRI trial was defined as at least 12 weeks of any of the following: ≥225 mg/day of clomipramine, ≥60 mg/day of fluoxetine, ≥60 mg/day of paroxetine, ≥200 mg/day of sertraline, ≥250 mg/day of fluvoxamine, ≥60 mg/day of citalopram, or ≥30 mg/day of escitalopram. An adequate trial was required so that patients had likely experienced the maximum benefit from SRI treatment before study entry (
21). At least minimal improvement (as reported by the patient, confirmed by the prescribing clinician when possible, and ascertained by the Clinical Global Impression improvement scale (
24)) was required because guidelines recommend that patients with no SRI response be switched to another SRI (
25). Patients who could not tolerate the aforementioned SRI doses were also eligible if they had at least minimal improvement at their maximally tolerated dose for at least 12 weeks. Concomitant medications were permitted if the dose was stable for at least 4 weeks prior to study entry and remained stable throughout the study.
Comorbid diagnoses were permitted if clearly secondary (i.e., the OCD symptoms were both the most severe and impairing). Patients were excluded for mania, psychosis, prominent suicidal ideation, substance abuse or dependence in the past 6 months, an unstable medical condition, pregnancy or nursing, or prior CBT while receiving an adequate SRI trial (≥15 sessions of either exposure and ritual prevention or stress management training within the past 2 months).
Eligibility was determined by skilled clinicians (psychiatrists or psychologists). Psychiatric diagnoses were confirmed by the Structured Clinical Interview for DSM-IV (
26). Treatment history was confirmed by the prescribing clinician and by chart review.
Randomization
Patients continuing SRI treatment were randomly selected for augmentation with CBT (either exposure and ritual prevention or stress management training) using a computer-generated stratified block randomization procedure that balanced the two CBT treatments for every four entrants at each study site (
27). Patients were informed of their CBT assignment by the study coordinator just prior to their first CBT session.
Medication
Patients initially met with a psychiatrist for 45 minutes and then monthly for 30 minutes for the purpose of maintaining a stable medication regimen so that changes in clinical condition could be attributed solely to CBT effects. Prescribing psychiatrists, who were blind to CBT assignment, offered encouragement and support but did not conduct CBT or insight-oriented psychotherapy. Medication adherence was assessed at each visit by verbal report and by pill counts. SRI blood level measurements were also obtained before and after CBT. SSRI levels were determined by liquid chromatographic methods (
28,
29) and clomipramine levels were determined by gas chromatography (
30).
CBT
Although different in content, the format of both CBT treatments was identical: 17 twice-weekly sessions (each 90–120 minutes), daily homework assignments, and between-session phone calls (twice per week, each <20 minutes).
Exposure and ritual prevention.
The protocol for exposure and ritual prevention followed the procedures of Kozak and Foa (
31). It included two treatment planning sessions and 15 exposure sessions, at least two of which occurred in the participant's home environment to promote generalization. Both in vivo and imaginal exposures were conducted, during which patients faced their fears for a prolonged period of time without ritualizing. Patients were asked to stop ritualizing after the first exposure session. The rationale provided to patients was that by experiencing exposure without rituals, they would learn that anxiety decreases with time alone (“habituation”) and that feared consequences do not occur. Although formal cognitive therapy procedures were not used, dysfunctional cognitions were discussed within the context of exposure (e.g., asking the patient, “Did you notice that your anxiety decreased without your ritualizing and nothing bad happened?”). As homework, patients were asked to record any rituals and spend at least 1 hour per day conducting self-guided exposures.
Stress management training.
Stress management training included procedures used by Lindsay et al. (
11) and was similar to stress inoculation training, a treatment effective in posttraumatic stress disorder and generalized anxiety disorder (
32–
34). Stress management training included two introductory sessions and 15 treatment sessions in which patients were taught stress management skills such as deep breathing, progressive muscle relaxation, positive imagery, assertiveness training, and problem solving. The rationale provided to patients was that life stressors can trigger OCD symptoms and that these stress management skills would reduce stress and thereby reduce OCD symptoms. As homework, patients were asked to monitor daily stressors and practice the stress management skills for at least 1 hour each day.
Assessments
Independent evaluators blind to CBT assignment evaluated patients at baseline (week 0), midway through CBT (after session 8/week 4), and after completion of CBT (after session 17/week 8). Symptom severity was evaluated using the Yale-Brown Obsessive Compulsive Scale (
35,
36) for OCD, Hamilton Depression Rating Scale (HAM-D) (
37) for depression, and Hamilton Anxiety Rating Scale (HAM-A) (
38) for general anxiety. At each assessment, patients also completed self-report measures of OCD severity (Obsessive-Compulsive Inventory—Revised (
39)), quality of life (Quality of Life Enjoyment and Satisfaction Questionnaire (
40)), and functioning (Social Adjustment Scale (
41)).
Quality control
Therapists providing CBT (nine psychologists and one psychiatrist) at each study site received training and supervision from faculty from the Philadelphia site (M.F. and E.H.) who had no other contact with study patients. Training included manual review and completion of at least one training case of each type under supervision. During the study, therapy sessions were audio- or videotaped and sent to supervisors for review; weekly group supervision for each treatment was held via teleconference. Four experienced CBT clinicians not otherwise involved in the study who were blind to outcome assessed the use of prescribed procedures in 25 randomly selected sessions of exposure and ritual prevention and 28 randomly selected sessions of stress management training. Therapists displayed excellent protocol adherence; for exposure and ritual prevention cases, 83% of exposure and ritual prevention (versus 0% of stress management training) procedures were used; for stress management training cases, 79% of stress management training (versus 6% of exposure and ritual prevention) procedures were used. Therapists rated patient adherence to homework assignments at each session on a 0 to 5 scale (where 0=did none of the homework and 5=did all of the homework). On average, patients displayed fair to good homework adherence (exposure and ritual prevention: mean=3.1, SD=0.9; stress management training: mean=2.6, SD=1.1; t=1.9, df=65, p>0.05).
Independent evaluators for each study site received training and supervision from faculty from the Philadelphia site (J.H.) who had no other contact with study patients; a manual outlined procedures for each measure. Independent evaluators met semiannually to review these procedures. To assess interrater reliability, a second independent evaluator listened to 30 taped diagnostic interviews; intraclass correlations were high (r=0.96, p<0.001).
Psychiatrists received training and supervision from a faculty member from the New York site (R.C.) following a manual outlining pharmacological procedures.
Statistical methods
To compare efficacy between treatment groups on continuous measures, outcomes at baseline, week 4, and week 8 were modeled as a function of time, treatment, and treatment-by-time interaction using linear mixed-effects models (
42) and SAS Proc MIXED (SAS Institute, Cary, N.C.). Time was treated as a continuous variable. Both the correlation between repeated measures and the selected autoregressive structure were based on Akaike's information criterion. Treatment group differences were assessed by the significance of the interaction term and the comparison of linear mixed-effects model estimates at endpoint (week 8). Site effects were assessed by including site in the linear mixed-effects models and by examining the interactions of site with treatment, time, and treatment-by-time. Response rates at week 8 were compared between groups using chi-square tests of independence; site effects were examined using the Mantel-Haenszel test. The primary outcome measure was the Yale-Brown Obsessive Compulsive Scale total score. Secondary measures were scores on the Obsessive-Compulsive Inventory—Revised (using the subscale with the maximum score (
43)), Social Adjustment Scale, Quality of Life Enjoyment and Satisfaction Questionnaire, HAM-D, and HAM-A and rates of response (defined as a ≥25% reduction in OCD severity as measured on the Yale-Brown Obsessive Compulsive Scale) (
44) and of achieving minimal symptoms (defined as a final Yale-Brown Obsessive Compulsive Scale score ≤12) (
44). All tests were conducted with two-sided significance levels (alpha=0.05). Effect sizes (Cohen's d) and 95% confidence intervals (CIs) were computed based on the observed data at week 8, using the difference between the means of the two types of treatment (stress management training–exposure and ritual prevention) divided by the pooled standard deviations for those means.
RESULTS
Recruitment and retention
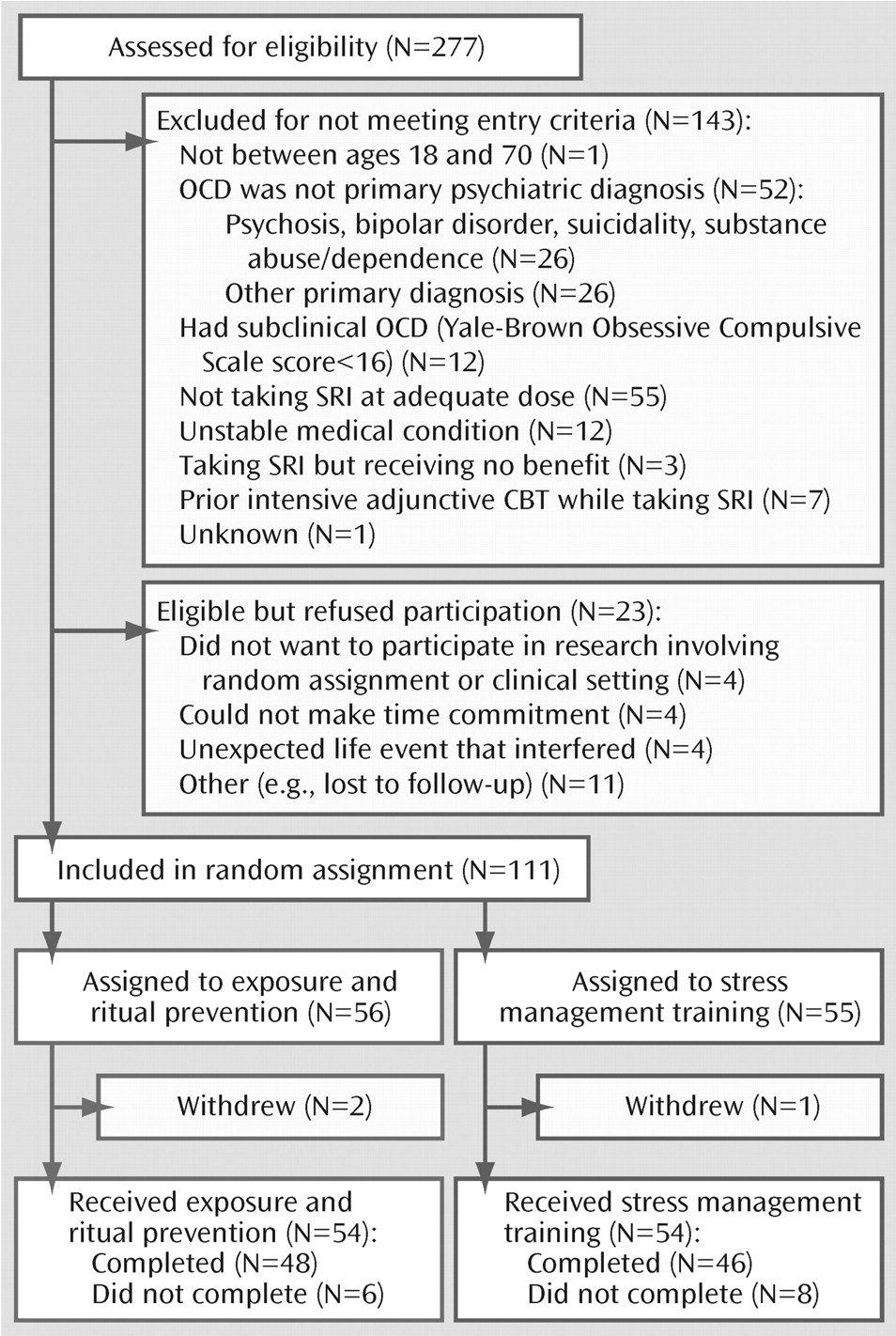
Of the 277 patients screened, 134 were eligible for study and 111 were randomly assigned to CBT augmentation (
Figure 1). Most patients were excluded (N=143) because OCD was not the principal diagnosis or they were not receiving (or willing to receive) an SRI at an adequate dose. Twenty-three eligible patients declined participation. The main reasons for declining participation were unwillingness to participate in research, lack of time, or interfering life events.
Of the 111 patients randomly assigned to CBT augmentation, 56 were assigned to exposure and ritual prevention and 55 were assigned to stress management training. Three patients (exposure and ritual prevention: N=2; stress management training: N=1) were subsequently withdrawn and excluded from all analyses because they were found not to meet inclusion criteria after randomization: one patient had never taken the prescribed SRI and two patients disclosed symptoms of comorbid disorders (bipolar disorder and anorexia) during treatment planning sessions that required immediate clinical attention.
Of the remaining 108 patients, 87% (N=94) completed CBT. No significant group differences emerged in dropout rates (exposure and ritual prevention: N=6 [11%]; stress management training: N=8 [13%]; χ2=0.3, df=1, p=0.57). Reasons for dropout included dislike of treatment (exposure and ritual prevention: N=1; stress management training: N=3); noncompliance with appointments (exposure and ritual prevention: N=2; stress management training: N=2); noncompliance with medication (exposure and ritual prevention: N=1); dislike of clinic setting (stress management training: N=1); unexpected life event (exposure and ritual prevention: N=1); and unknown (exposure and ritual prevention: N=1; stress management training: N=2).
Sample characteristics
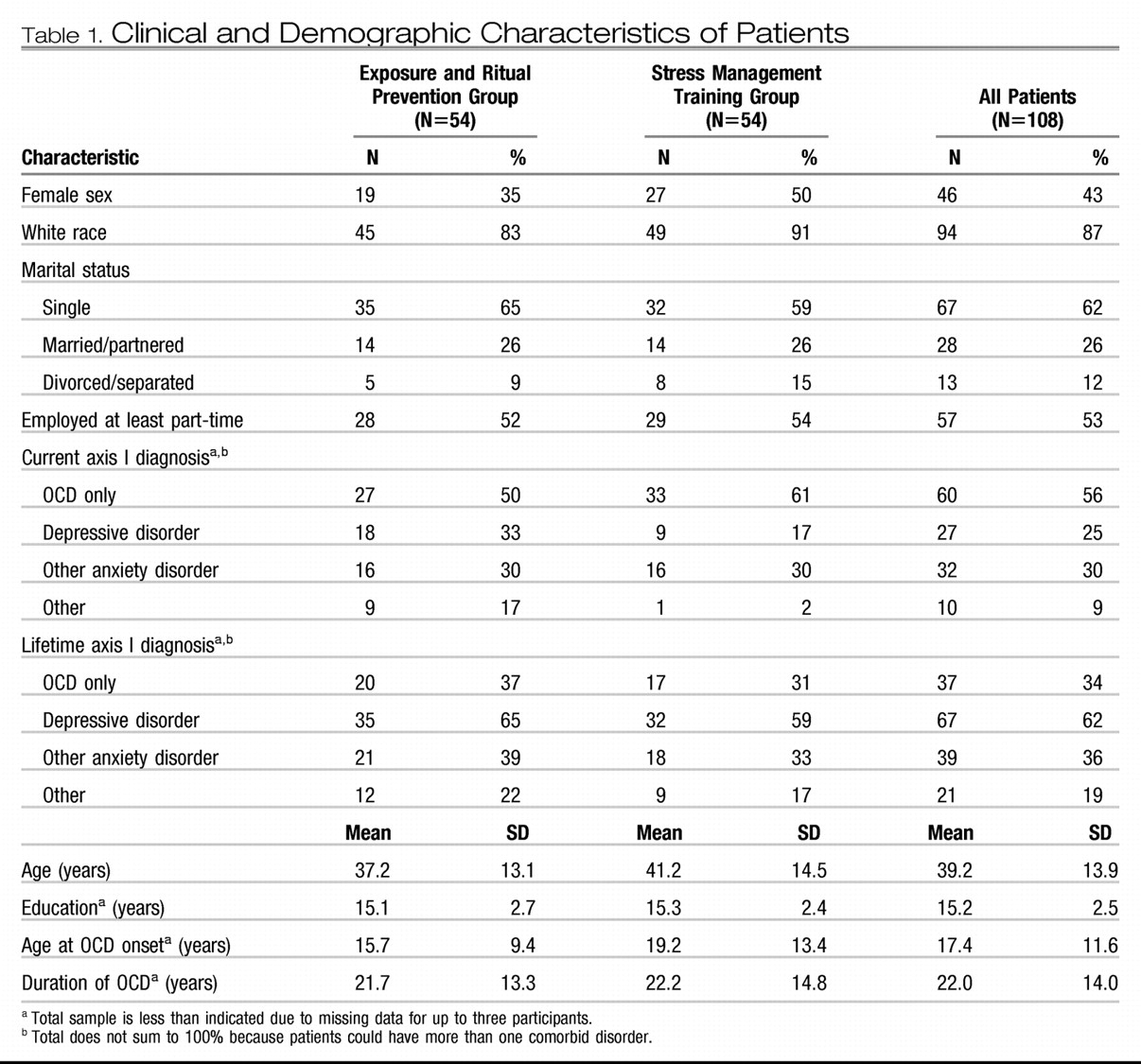
Pretreatment demographic and clinical characteristics are presented in
Table 1. Only one significant treatment group difference emerged: patients receiving exposure and ritual prevention had a higher proportion of “other” current comorbid disorders (χ
2=6.9, df=1, p=0.009).
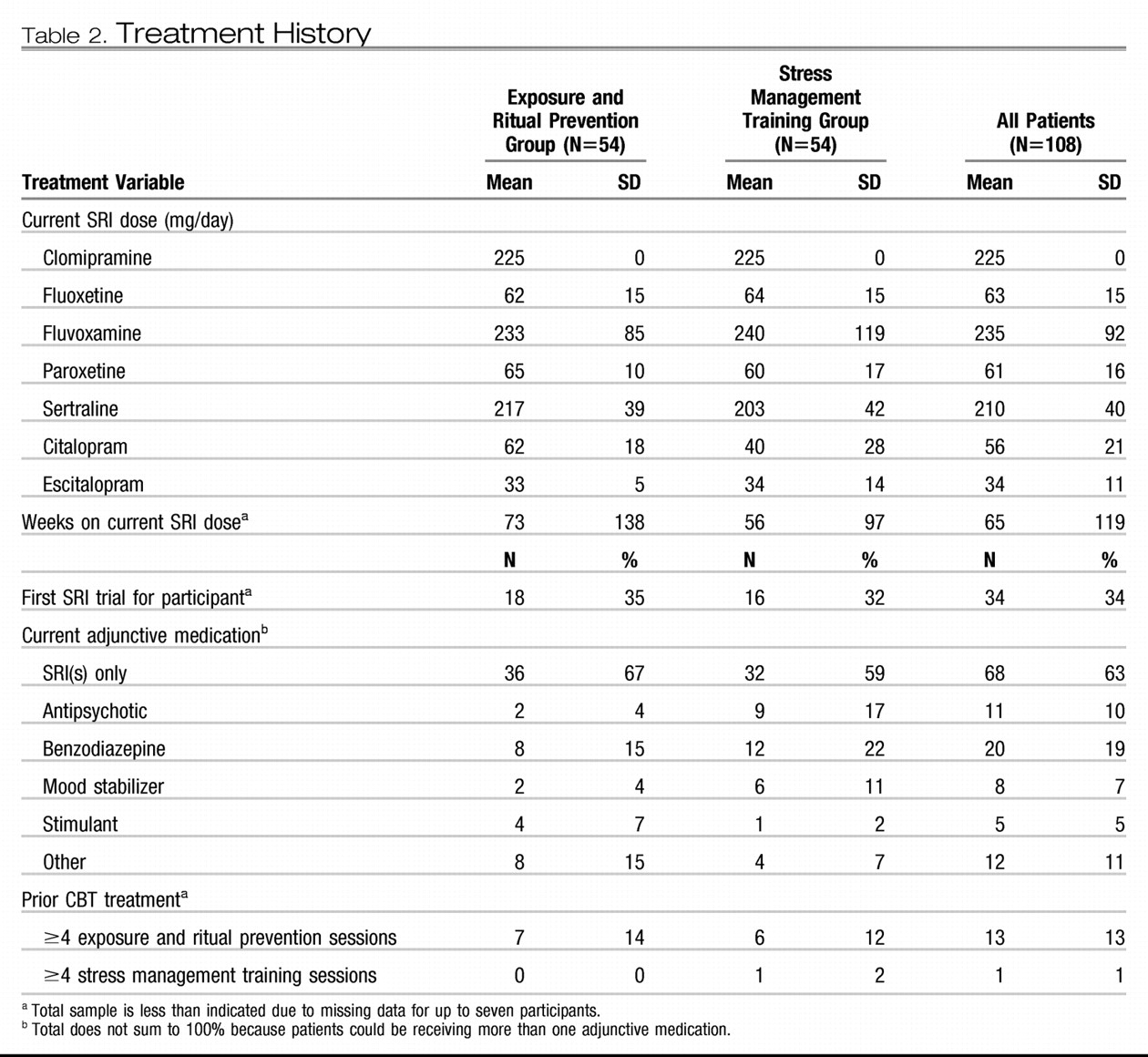
Treatment history is presented in
Table 2. All patients were currently taking an SRI; 8% (N=9) were currently taking two SRIs. Most patients had been receiving an adequate SRI dose for more than the required minimum of 12 weeks (78% for at least 16 weeks and 50% for at least 24 weeks). Some patients (37%) were taking concomitant medications, most commonly a benzodiazepine. Few had received any prior exposure and ritual prevention or stress management training. Only one significant group difference emerged: patients randomly assigned to stress management training were more likely to be receiving concomitant antipsychotics (χ
2=4.9, df=1, p=0.03).
Pharmacotherapy
One patient receiving exposure and ritual prevention stopped taking the SRI at week 3 and was removed from the study from that point forward. All others reported continuing a stable SRI dose while receiving CBT. Seventy-five patients (exposure and ritual prevention: N=38; stress management training: N=37) had SRI blood levels measured before and after CBT; these levels showed little change (intraclass correlation=0.975, CI=0.96–0.98). No serious SRI adverse events emerged during CBT treatment.
Efficacy of CBT augmentation
Primary outcome.
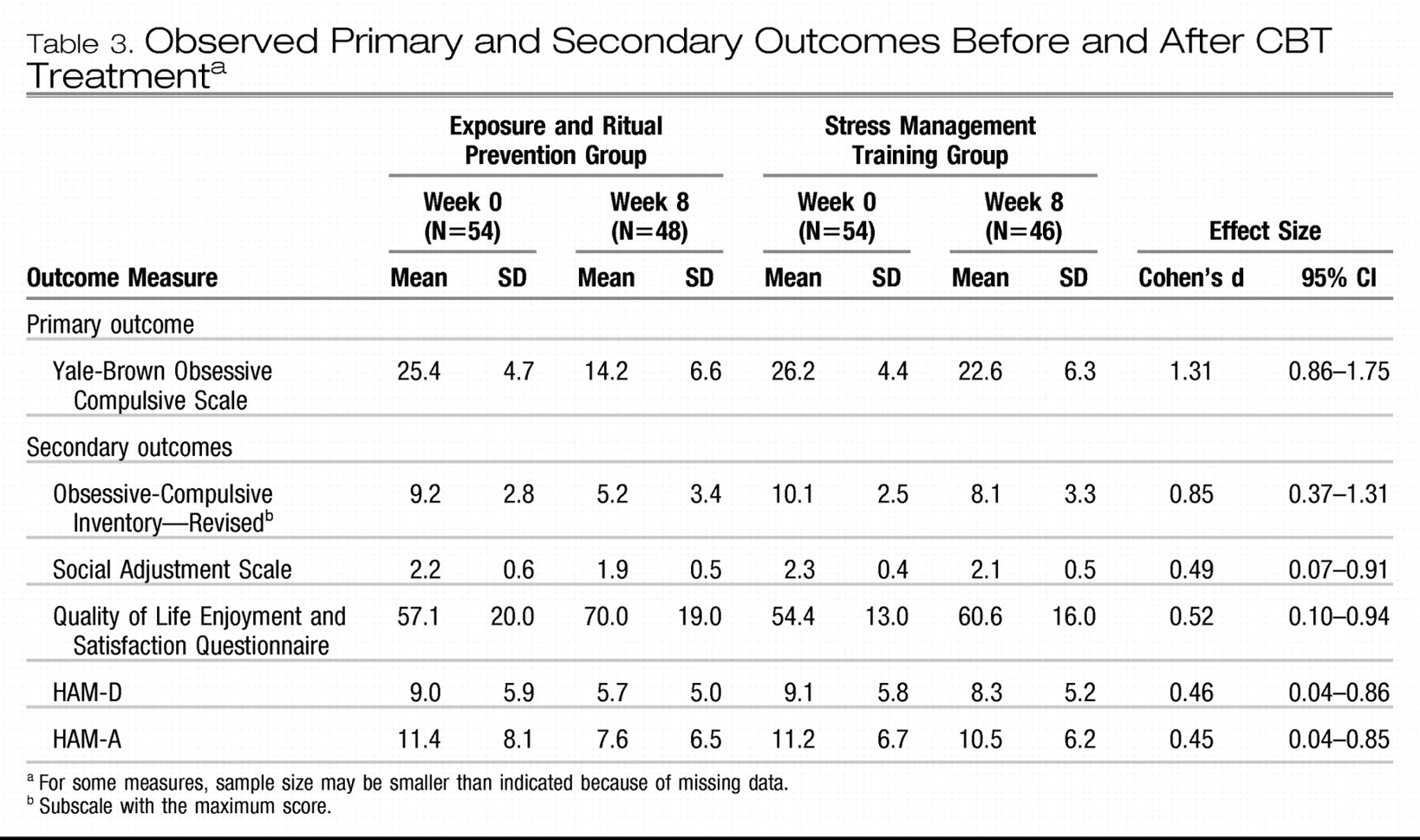
Observed mean Yale-Brown Obsessive Compulsive Scale scores and effect sizes are presented in
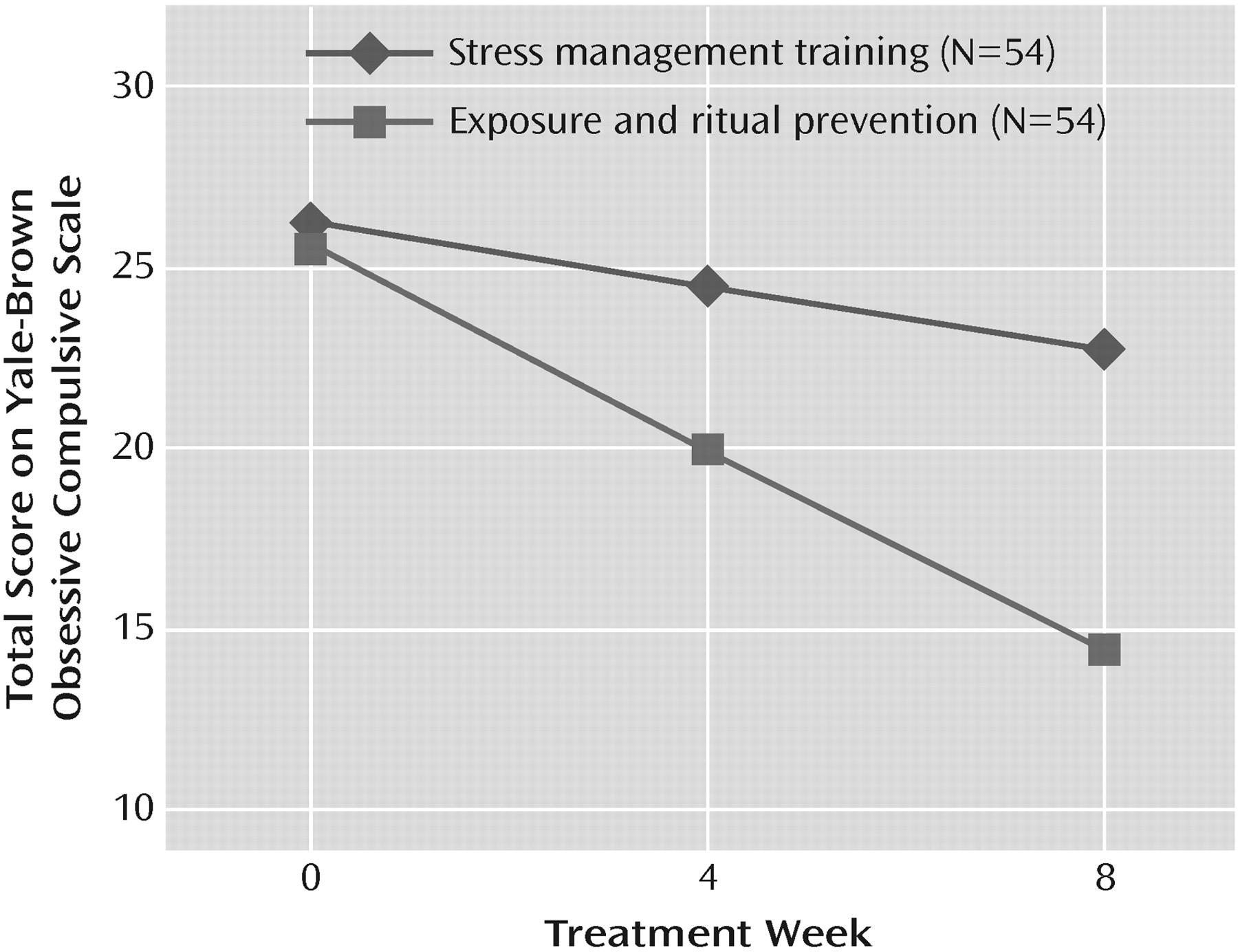
Table 3. Time-by-treatment interaction in the linear mixed-effects model for the Yale-Brown Obsessive Compulsive Scale was significant (F=40.1, df=1, 187, p<0.001), indicating greater symptom reduction in the group receiving exposure and ritual prevention (average loss of 1.4 scale points per week [SE=0.1]) compared with the group receiving stress management training (0.4 scale points per week [SE=0.1]) (
Figure 2). After 17 sessions of CBT (week 8), patients receiving exposure and ritual prevention also had significantly lower Yale-Brown Obsessive Compulsive Scale scores than patients receiving stress management training based on linear mixed-effects model estimates (14.3 [SE=0.9] versus 22.7 [SE=0.9]; F=41.9, df=1, 187, p<0.001).
Secondary outcome.
Observed mean scores and effect sizes for secondary outcome measures are presented in
Table 3. Time-by-treatment interactions in the linear mixed-effects models showed greater symptom decrease over time with exposure and ritual prevention than with stress management training on the Obsessive-Compulsive Inventory—Revised (F=9.6, df=1, 160, p=0.002) and the HAM-A (F=4.1, df=1, 187, p=0.04), but not on other measures (HAM-D: F=2.4, df=1, 187, p=0.12; Social Adjustment Scale: F=1.5, df=1, 176, p=0.22; Quality of Life Enjoyment and Satisfaction Questionnaire: F=2.5, df=1, 175, p=0.11). Allowing for a lenient interpretation of interactions on secondary measures (
45), we examined differences at week 8 and found superior outcome for exposure and ritual prevention on all measures (Obsessive-Compulsive Inventory—Revised: F=17.2, df=1, 160, p<0.001; HAM-A: F=4.5, df=1, 187, p=0.04; HAM-D: F=3.9, df=1, 187, p=0.05; Quality of Life Enjoyment and Satisfaction Questionnaire: F=6.2, df=1, 175, p=0.01; Social Adjustment Scale: F=5.1, df=1, 176, p=0.03).
Significantly more patients receiving exposure and ritual prevention than patients receiving stress management training achieved responder status (74% [CI=62–86] versus 22% [CI=11%–33%], respectively; χ2=29.1, df=1, p<0.001; phi=0.52). The number needed to treat for responder status was 2 (CI=1–3). Significantly more patients receiving exposure and ritual prevention than patients receiving stress management training also achieved minimal symptoms (33% [CI=20%–46%] versus 4% [CI=−1% to 9%], respectively; χ2=15.7, df=1, p<0.001; phi=0.38). The number needed to treat for minimal symptoms was 4 (CI=2–6). Clinical vignettes of a patient who achieved minimal symptoms after exposure and ritual prevention and of a patient who did not respond to stress management training are presented in the Patient Perspectives.
Site effects
There were two significant site differences in baseline demographic and clinical characteristics (
Table 1 and
Table 3). Compared to patients from the New York site, patients from the Philadelphia site were more likely to be receiving their first SRI trial (47% [N=22] versus 22% [N=12]; χ
2=6.8, df=1, p=0.009). Patients from the Philadelphia site also had a higher pretreatment score on the Obsessive-Compulsive Inventory—Revised (10.3 [SE=2.3] versus 9.1 [SE=2.9]; t=2.3, df=94, p=0.02).
There was a significant site-by-treatment-by-time interaction on Yale-Brown Obsessive Compulsive Scale scores (F=4.8, df=1, 187, p=0.03). Although the difference between exposure and ritual prevention and stress management training was significant at each site (all p values <0.01), the rate of decrease in symptom severity with exposure and ritual prevention was faster at the Philadelphia site; the rate of decrease in symptom severity with stress management training was similar at both sites. Mantel-Haenszel tests indicated that the sites did not differ in rates of response or of achieving minimal symptoms for either treatment (χ2<0.5, df=1, all p values >0.50). No other interactions with site approached significance (all p values >0.08).
DISCUSSION
The addition of exposure and ritual prevention reduced OCD symptom severity more than the addition of stress management training in patients with clinically significant OCD despite an adequate SRI trial. Also, more patients who received exposure and ritual prevention were treatment responders and achieved minimal symptoms. Our study extends the findings of Tenneij et al. (
17) by including a psychosocial comparison group, patients with moderate to severe OCD symptoms, and patients with multiple comorbidities. Because we used a controlled design and patients received an adequate SRI dose for at least 12 weeks prior to study entry, we were able to attribute the observed benefits solely to the specific effects of exposure and ritual prevention. Consistent with prior studies (
11,
12), stress management training had little effect on OCD symptoms, and the data suggest that continuing an SRI alone for 8 additional weeks would not be beneficial. Together, these findings strongly support the use of exposure and ritual prevention as an SRI augmentation strategy for OCD.
Although augmentation with exposure and ritual prevention was effective, our patients did not fare as well as other study cohorts who received intensive exposure and ritual prevention (15 daily exposure sessions over 3 weeks) as an initial treatment but not SRIs (
9). Two factors might contribute to this difference. First, our patients had moderate to severe OCD (baseline Yale-Brown Obsessive Compulsive Scale score of ∼25) despite an adequate SRI trial. Such patients may be less responsive to treatment in general. Second, achieving minimal symptoms in patients with significant symptoms despite an adequate SRI trial may require more than 15 exposure sessions or intensive treatment.
While patients receiving exposure and ritual prevention did not differ from patients receiving stress management training in the rate of change in quality of life or functional impairment, post-hoc analyses revealed modest but significantly superior functioning and quality of life at week 8. Larger improvements in functioning and quality of life may require that patients complete treatment with minimal OCD symptoms. Alternatively, improvements in functioning and quality of life may lag behind improvement in OCD symptoms.
Study limitations
Several design features merit consideration. First, to enhance feasibility and mimic what clinicians encounter in practice, we recruited patients who had already received an adequate SRI trial. Consequently, we had no objective measures of symptom severity prior to the SRI trial or of SRI response, although all participants reported experiencing at least minimal improvement after receiving SRIs. Second, because all participants reported at least minimal improvement after receiving SRIs, our results may not apply to SRI-refractory patients; however, previous open trial data (
15) suggest that these patients can also benefit from augmentation with exposure and ritual prevention. Third, we used a twice-weekly CBT format. Weekly sessions are more practical, but their effectiveness in this context requires further study. Fourth, although our patient adherence measures are standard (pill counts and blood levels for SRIs and therapist records of homework adherence for CBT), they have not been psychometrically validated. Validating adherence measures for exposure and ritual prevention and examining the relationship between adherence and outcome are important areas for future research.
Site effects
Patients receiving exposure and ritual prevention at the Philadelphia site (the expert site for exposure and ritual prevention) had significantly greater rates of change in OCD symptoms than patients receiving exposure and ritual prevention at the New York site. However, this did not lead to significant site differences in mean Yale-Brown Obsessive Compulsive Scale scores nor in rates of response or of achieving minimal symptoms after 8 weeks of treatment. The absence of clinically meaningful site effects was likely due to the careful training and supervision provided by the faculty from the Philadelphia site. These data suggest that an effective way of disseminating exposure and ritual prevention could involve training followed by weekly or biweekly expert group supervision, as was done here. A similar dissemination model has been used successfully in Norway (
46).
Generalizability
Our study was designed to recruit patients similar to those seen in routine clinical practice. Thus, exclusion criteria were minimized and reflected good clinical practice. As a result, many patients had comorbid anxiety and depressive disorders (despite SRI treatment), as is typical in clinical practice (
47,
48). Moreover, the most common reason for exclusion was that the patient was not taking (or willing to take) an SRI at an adequate dose. Thus, we believe our findings are broadly applicable to OCD patients on SRIs who seek to augment their treatment with exposure and ritual prevention.
The encouraging outcome of exposure and ritual prevention observed in our study is consistent with studies conducted elsewhere that used similar exposure and ritual prevention procedures, including specialty fee-for-service practices (
49,
50) and anxiety research clinics in the United States (
16) and Europe (
17,
51). Moreover, a recent study from Norway (
46) found that even therapists in non-academic community clinics can achieve good outcomes with training and group supervision by experts in exposure and ritual prevention. These data suggest that our results can be generalized to other clinical settings.
Implications for care
Treatment guidelines for OCD do not recommend combining SRIs and exposure and ritual prevention in all patients (
2). The reason is that combination treatment has not always been superior to monotherapy in randomized, controlled trials that introduced the two therapies simultaneously. In the present study, we found that providing exposure and ritual prevention to patients already receiving an adequate SRI dose led to a reduction in OCD symptoms in most patients. Thus, although combination treatment may not be necessary for all OCD patients, sequencing these treatments as we did may help many patients for whom SRI treatment alone is not enough.
There are now two evidence-based strategies for augmenting SRI response in OCD: the addition of exposure and ritual prevention or the addition of antipsychotic medication (
5). Comparisons across studies suggest that 8 weeks of twice-weekly exposure and ritual prevention is as efficacious as 6 to 8 weeks of antipsychotic augmentation. Exposure and ritual prevention is safer than antipsychotics, given their known risks (e.g., tardive dyskinesia, neuroleptic malignant syndrome, and metabolic syndrome) (
52). However, exposure and ritual prevention is not as widely available, and we do not know which strategy patients prefer. Importantly, neither 17 sessions of exposure and ritual prevention over 8 weeks nor up to 8 weeks of antipsychotic augmentation is sufficient to help most OCD patients with clinically significant symptoms despite an adequate SRI trial to achieve minimal symptoms. We are currently conducting a randomized, controlled trial that directly compares the efficacy and durability of exposure and ritual prevention and antipsychotic augmentation to address these issues.
Acknowledgments
Dr. Simpson is an advisor to Anxiety Disorders Association of America and Jazz Pharmaceuticals and has received medication at no cost from Janssen Pharmaceutica for a study. Dr. Foa has received research support from Pfizer, Solvay, Eli Lilly, SmithKline Beecham, GlaxoSmithKline, Cephalon, Bristol-Myers Squibb, Forest, Ciba-Geigy, Kali-Duphar, and APA; she has been a speaker for Pfizer, GlaxoSmithKline, Forest, and APA; and she receives royalties from the sale of two books on obsessive-compulsive disorder from Bantam and Harcourt. Dr. Liebowitz has received research support from Eli Lilly, Wyeth, GlaxoSmithKline, Avera, Novartis, Pfizer, AstraZeneca, Tikvah Therapeutics, Forest, Sepracor, Horizon, and Johnson and Johnson; he has served as a consultant for Eli Lilly, Avera, AstraZeneca, Pherin, Wyeth, Jazz Pharmaceuticals, Zars Pharma, and Tikvah Therapeutics; and he has served on the speakers bureau for Wyeth and Bristol-Myers Squibb. In addition, Dr. Liebowitz has licensing agreements for rating scale and/or electronic data capture devices with GlaxoSmithKline, Pfizer, Avera, Eli Lilly, Indevus, Tikvah Therapeutics, and Servier; he holds the copyright of the Liebowitz Social Anxiety Scale; and he holds equity ownership in the Medical Research Network (a private research site) and ChiMatrix (a company specializing in electronic data capture).
All remaining authors report no competing interests.