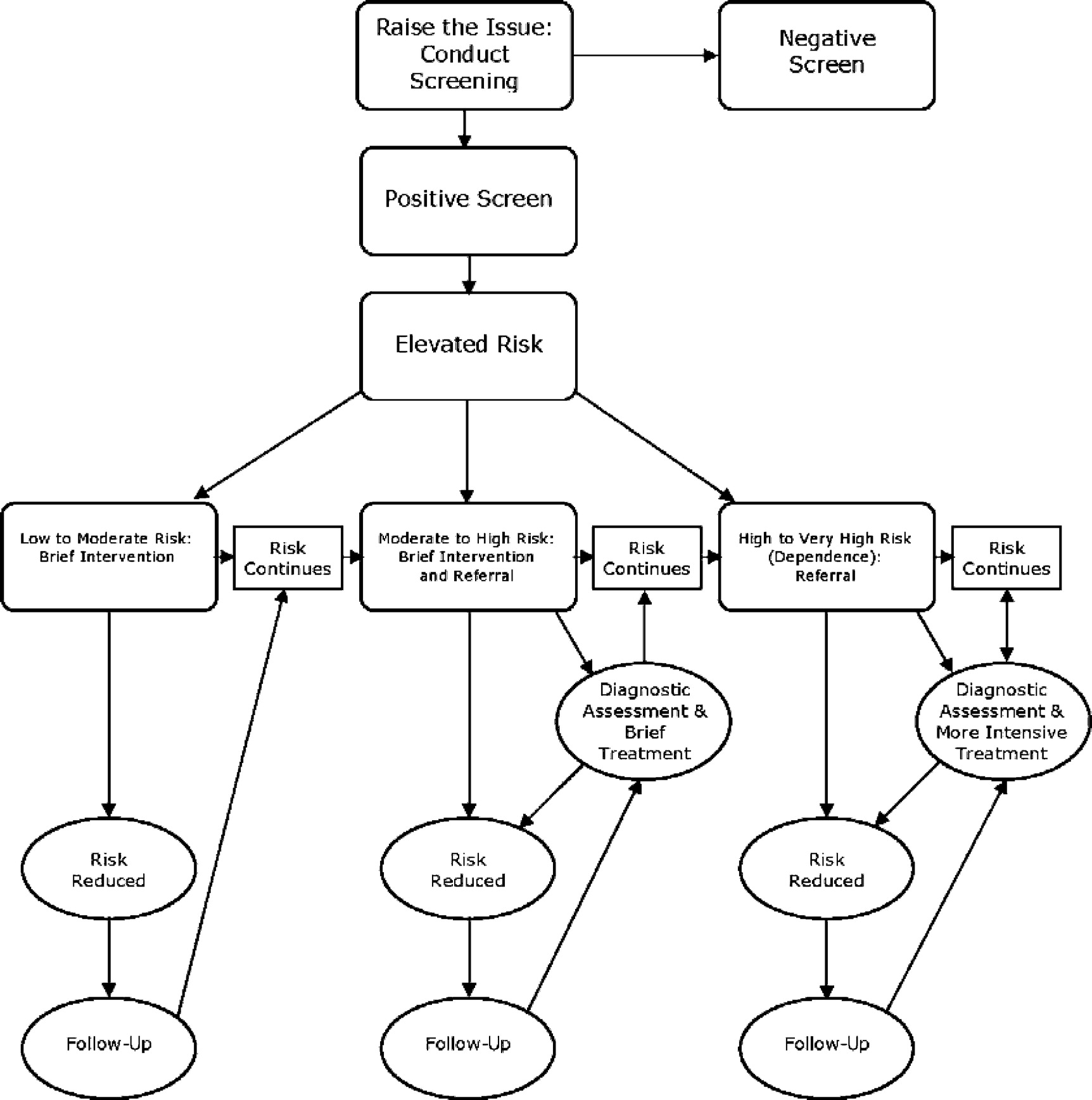
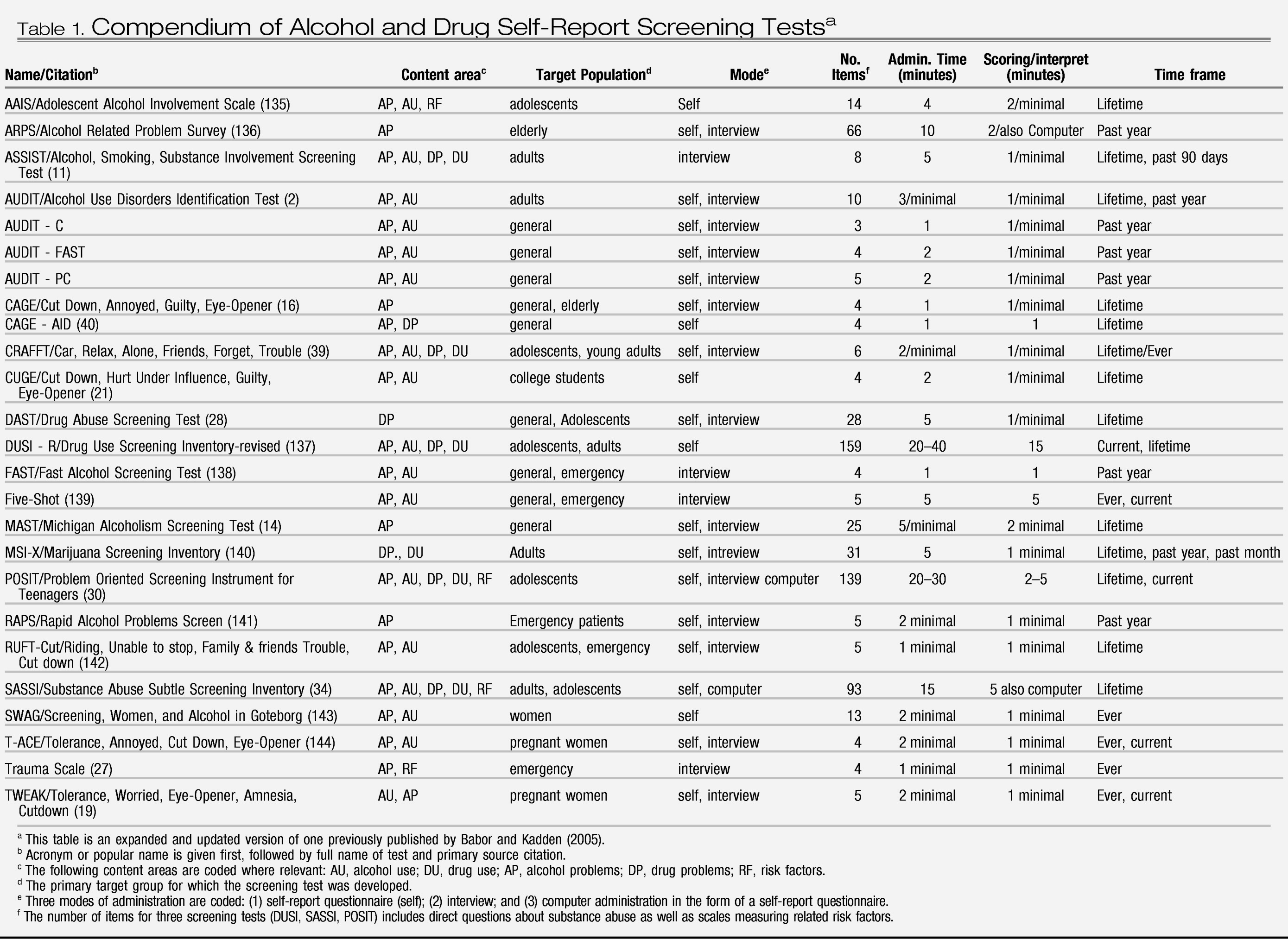
There are several important economic issues to consider in relation to implementing SBIRT. Providers, financial managers, and decision makers need accurate information about the costs of screening and brief interventions and estimates of the revenue potential. Decision makers also need to know the cost-effectiveness of various SBIRT models in order to choose between lower cost/less effective models and higher cost/more effective models. Cost benefit estimates are needed to assess the net costs to health plans or to society of diverting resources to SBIRT activities. In this section we summarize research on each of these issues.
Cost.
SBIRT costs will vary, depending on the perspective from which costs are calculated, e.g., the provider's, the payer's, the patient's, or society's perspective. For financial management purposes, the total costs of SBIRT services can be broken down into their components, e.g., screenings, information packets, counseling sessions, and case management. From the provider's perspective the cost of brief interventions depends primarily on the nature and severity of the client's alcohol or drug problems, the number of sessions that comprise the interventions, the personnel delivering brief interventions, the resources to produce and deliver interventions (and treatments) and the settings in which brief interventions are provided. Providers must also consider the one-time costs of developing and starting the service plus any on-going continuing costs such as continuing education of staff. From the client's perspective, the cost of SBIRT includes the amount the client pays for the intervention beyond the premiums for health insurance, as well as time and transportation costs to the site where interventions are furnished. From a payer's perspective, the cost of brief interventions might be defined as the amount paid for the service minus any financial benefit that may accrue from the reduction of future costs resulting from the service. From society's perspective, the cost of brief interventions is expressed in terms of the market value of the best alternative use to which labor, capital, and other resources may be put (i.e., economic or opportunity costs).
Given the various perspectives that could be used, it is not surprising that published estimates of the costs of SBIRT vary considerably. For example, Zarkin et al. (
126) estimated screening costs at $0.42 per patient for a 2-minute screen versus $16 per patient by Gentilello et al. (
127) and $497 per patient by Kunz et al. (
128). Given the fact that fewer than 30 percent of patients screened are referred for brief interventions, efforts to reduce the initial screening costs can significantly reduce the overall cost of providing alcohol SBI. There is also broad variability in the costing methodology used in the literature. For example, brief intervention costs have been reported at $2.59 per patient (
126), $135 per brief intervention session (
128), and $0.59 median per member per month (insurance premium cost) (
129). Obviously, the underlying variability of the SBI programs is a primary cause for the variation in cost estimates, but the lack of a consistent costing methodology contributes to the variability and limits the usefulness of cross-program comparisons.
Furthermore, current SBI cost-effectiveness and cost-benefit research often presents cost results without a detailed description of the costing methods used. Many of these studies do not adequately address how and what was actually costed (e.g.,
130). Authors often take national wage averages and estimate the amount of time for services (e.g., 127). The most thorough cost estimate comes from the Cutting Back study (
126), which used activity-based costing to separate start-up costs from ongoing implementation costs, a distinction overlooked by previous studies. Cutting Back is the only study to compare costs across providers and is also the first to cost different models of implementation. However, the SBI models studied by Zarkin et al. (
126) were implemented exclusively for the Cutting Back research project, and therefore the authors were forced to make judgments as to which costs would likely be retained in a non-research setting.
Cost effectiveness analysis:
Cost effectiveness analysis (CEA) enables decision makers to compare the economic merits of alternative types of service, such as brief interventions and standard care, which represents the care that clients would ordinarily receive. Kunz et al. (
128) found cost-effectiveness ratios for brief interventions administered in a hospital emergency department of $258 for a one unit reduction in the follow-up AUDIT score, $219 for a decrease of one drink per week, and $61 for a one percentage point decline in the follow-up probability of heavy drinking. In a study that applied estimates from published studies to Australia, Wutzke et al. (
130) found that brief physician advice to at-risk drinkers resulted in additional years of life from fewer accidents. Dividing the cost of the intervention by the number of life-years saved yielded a cost of approximately Aus $1,873 per life-year saved. CEA does not, however, provide definitive recommendations on which program should be adopted. Rather, it provides decision makers with evidence on the relative benefits and costs of one program compared to another. For this reason, CEA alone is often not enough to justify adoption of a new program.
Cost benefit analysis:
Unlike CEA, CBA places a dollar value on all outcomes and directly compares to the dollar value of a program's outcomes to the dollar value of its costs. As a result, CBA often provides definitive answers on which programs should be adopted. The program with the largest dollar benefit after accounting for costs should unambiguously be adopted. There are various methods with which to compare the benefits of a program to its costs, including: net benefit measures in which costs are subtracted from benefits; return on investment in which the benefits are expressed as a percentage return to the investment represented by the program costs; and the benefit cost ratio in which benefits are expressed as a ratio of the costs. The choice of CBA measure is largely determined by the audience, with return on investment often appealing more to business or corporate audiences and net benefit or benefit-cost ratios appealing more to academic audiences.
The CBA evidence on SBIRT is generally very favorable. In a randomized trial of brief interventions administered in physician offices, Fleming and colleagues (
131) found that a group receiving a brief intervention not only had significant reductions in alcohol use, they also had fewer hospital days and fewer emergency department visits. The intervention cost $205 per person ($166 from the clinic perspective and $39 from the client's perspective) and saved $712 in health care costs. The benefit cost ratio of 4.3 suggests a $43,000 savings in future health care costs for every $10,000 spent for early intervention. The benefit cost ratio increased to 39:1 after factoring fewer motor vehicle and legal events into the analysis.
In a CBA using published sources, Gentilello et al. (
127) estimated that the screening and brief alcohol interventions provided to injured patients treated in an emergency department or admitted to a hospital together cost $54 per patient, or $16 plus $38, respectively. The net cost savings from the screening and intervention was estimated at $89 per patient, or $330 for each patient receiving an intervention (27 percent had a positive screen). The benefit, in the form of reduced direct health costs, resulted in a savings of $3.81 for every $1.00 spent on screening and intervention, for a benefit cost ratio of 3.8:1. If interventions were routinely offered to injured adult patients nationwide, it was estimated that the potential net savings might approach $1.82 billion annually.
In a retrospective study of admissions to the Naval Medical Center in Portsmouth, Virginia, Storer (
132) estimated that intervention patients had significantly lower hospital readmission rates than other patients. The lower readmission rate for intervention patients alone generated an estimated savings of $606,400, for a total cost of $31,500 (benefit cost ratio of 19:1), for an average cost of $154 for 205 brief interventions.