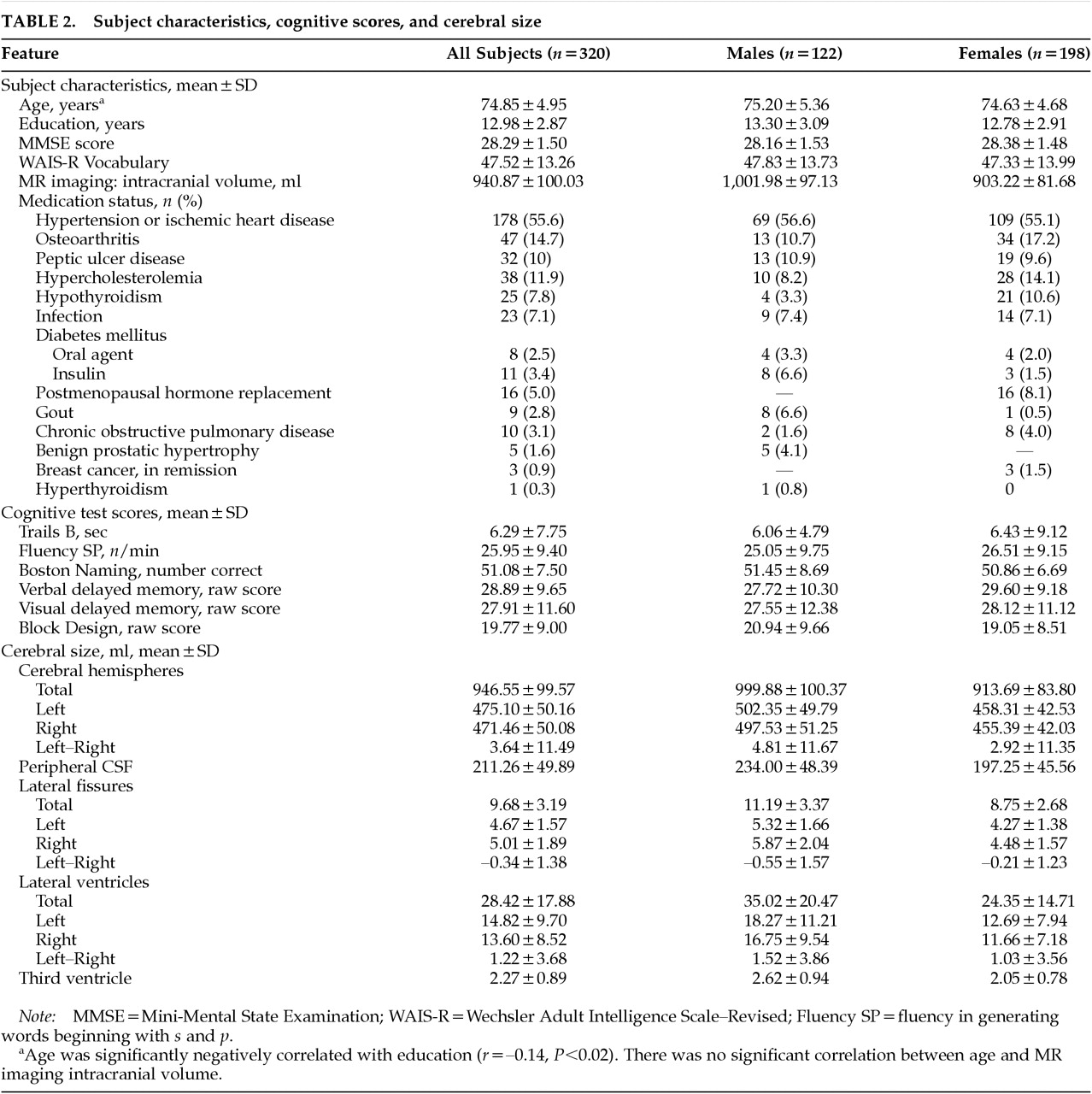
In this large sample of 320 elderly individuals living independently in the community, increased age was associated with cerebral atrophy and ventricular enlargement, as well as with decreased performance on tests of attention and psychomotor speed, memory, and visuospatial construction. Our study has the advantage of a relatively large sample selected from community-dwelling participants who did not present for medical care, and, as such, our results are more likely to be representative of the community. In addition, our blinded measures of brain structure were highly reliable, and our estimates of their age-specific sizes agree closely with previous reports, including those using more sophisticated voxel-by-voxel tissue classification techniques.
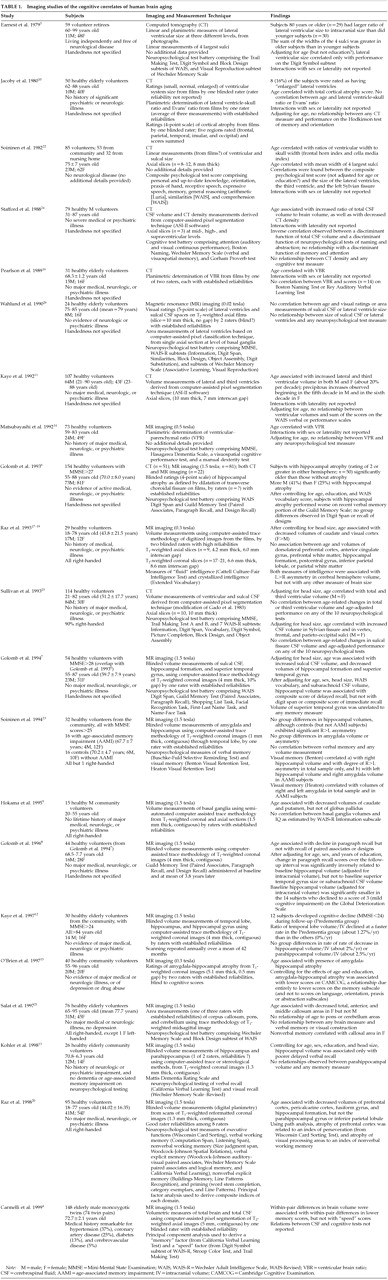
1 Our findings of age-related reductions in cognitive test performance are also consistent with the extant literature.
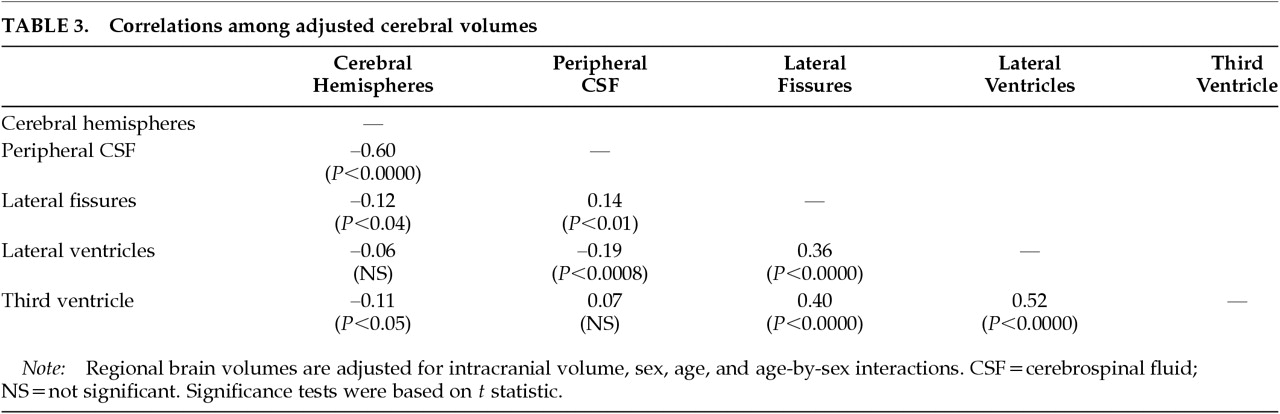
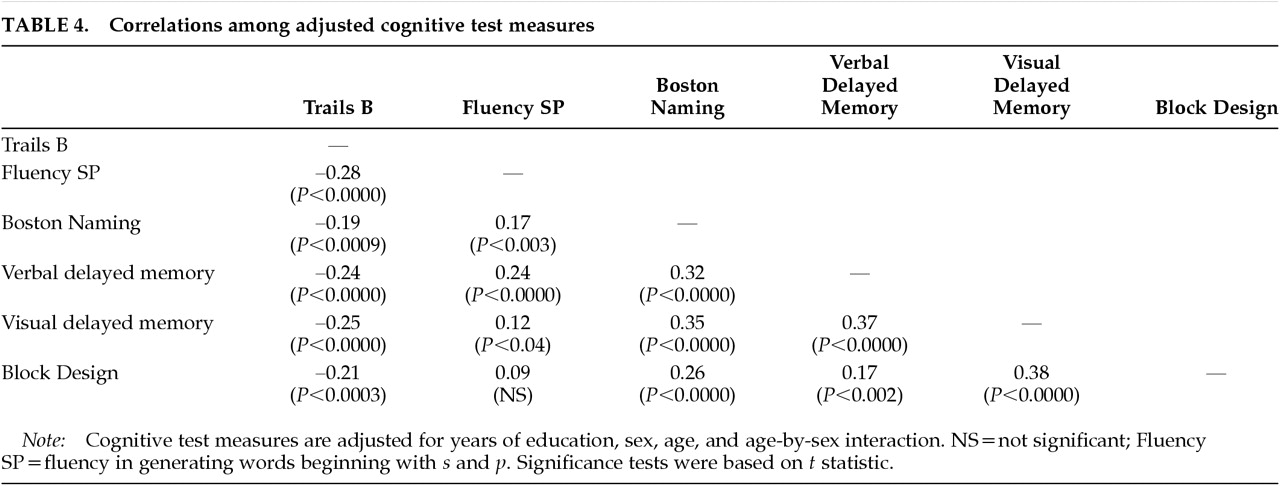
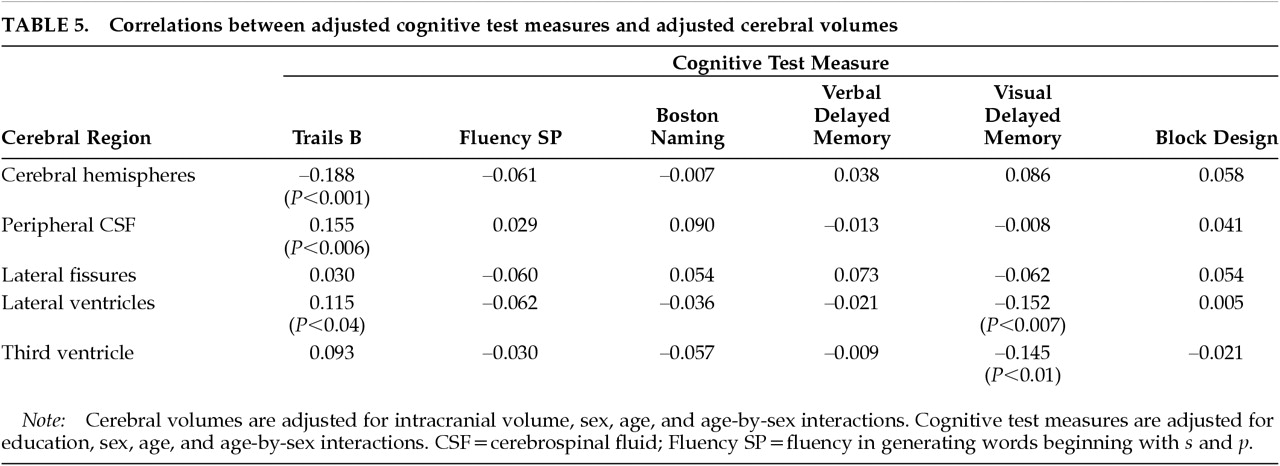
2,3The age-specific changes in cerebral size were associated with poorer performance on two of the cognitive test measures. First, cerebral atrophy (as measured by both decreased cerebral hemisphere volume and by increased peripheral CSF volume) and lateral ventricular enlargement were each associated with poorer performance on Trails B, a measure of attention, psychomotor speed, and working memory. Second, enlargement of the lateral ventricles and the third ventricle was associated with poorer visual delayed memory. Our interpretation of a complex pattern of correlations among brain structure and cognitive test variables was facilitated by the use of path analysis. Path analysis provided the simultaneous multivariate estimation of the adjusted brain volumes, the adjusted test scores, the mutual correlations among the adjusted brain volumes, the mutual correlations among the adjusted test scores, and the correlations between adjusted brain volumes and adjusted test scores.
Methodological Limitations
Our findings are subject to certain potential limitations. Although cross-sectional studies of age effects allow for relatively efficient and rapid acquisition of large amounts of data, they are subject to secular effects such as birth cohort.
46 This effect refers to the possibility that brain size, like cranial size, may exhibit systematic changes over successive birth cohorts in the general population. If such trends actually exist in the population at large and if they are not secondary to secular trends associated with correlates such as cranial size (note that in the present study intracranial volume was not correlated with age), then an assessment of the true effects of aging per se on brain volume will require longitudinal investigation.
A second issue relates to the health status of our subjects. Our sample represents a group that may be somewhat healthier than the entire elderly population because of selection criteria for the CHS and the current study.
28,29 As such, our findings may not be applicable to the entire population of seniors. There is also heterogeneity of health status within our subjects, in that 29% were free of major systemic illness and 71% had at least some mild physical disease, corresponding to the distinction between successful and usual aging.
52 Such differences in health status could account for differences in brain aging, and indeed systemic disease such as hypertension has been found to be associated with changes in brain structure.
42,53,54 In our sample, however, the presence of hypertension/ischemic heart disease was not associated with brain size (Wilks' lambda=0.95, df=13,306,
P=0.26). Finally, it is possible that at least some subjects in our sample may have experienced undetected cognitive decline despite our screening and exclusionary criteria. Still, our use of an MMSE cutoff score of 24 is widely accepted in the literature
35 and represents an appropriate strategy for selection of a sample that is representative of the community-resident “usual aging” population.
Third, there exists the possibility of a Type I error in our findings because many correlations were tested. Thus, our analyses must be considered exploratory, and our finding of a relationship requires confirmation by additional hypothesis-testing research.
Fourth, the tests used in this study constituted part of a larger test battery that was originally chosen to be maximally sensitive to aging rather than for its potential to provide information about the neuroanatomic basis of cognitive function. Many tests that are sensitive to aging, such as Trails B, are thought to be measures of functions (like information processing speed) that may be more diffusely represented in the brain. Therefore, the original purpose for the battery of tests used in this study may have led to the selection of instruments that are sensitive to global age-related changes but less suitable for the investigation of focal brain–behavior relationships. Consequently, tests such as Trails B may be particularly likely to correlate with the global anatomic variables reported in this study but less likely to be related to focal, lateralized brain changes.
Finally, the measurements of cerebral size in our study are subject to certain limitations.
1 First, because of limitations inherent in the CHS MR imaging acquisition protocol, our analyses of cerebral size were restricted to the axial plane; three-dimensional reconstruction was not possible without dramatic loss of resolution. Second, accurate delineation of regional boundaries can be affected by several sources of technical error, including improper window center settings, magnetic field inhomogeneity (resulting in spatial distortion of objects and object pixel nonuniformity), and differences in MR imaging technical variables.
43 The effects of these variables were minimized in this study by using a set of procedures that has been shown to optimize the accuracy of MR imaging size measurements.
43,47 Third, field strength differences between the two scanners could affect estimates of brain size.
1 Including scanner assignment in the path analysis did not, however, alter any of the correlations between cerebral volumes and neuropsychological test measures in our study.
Cognitive Correlates of Age-Specific Changes inCerebral Size
The following cognitive correlates with age-specific changes in cerebral size emerged from the present study:
Attention and Psychomotor Speed. We found that the two hallmarks of brain aging, cerebral atrophy (as measured both by decreased cerebral hemisphere volume and increased peripheral CSF volume) and ventricular enlargement, were each associated with poorer performance on the Trail Making Test, a measure of attention, psychomotor speed, and working memory. Our results are consistent with those of Earnest et al.,
5 who observed that ventricular enlargement on CT was associated with poorer performance on the Digit Symbol subtest of the WAIS-R, a measure of attention and psychomotor speed (
Table 1). In contrast, most of the extant literature reports no relationship between measures of attention and psychomotor speed and either cortical atrophy
4,5,24–26 or ventricular enlargement,
5,25 including studies using the Trail Making Test (
Table 1).
4,5,25,26 These negative findings are difficult to compare with the current study, however, because of differences in subject sample (age range, sex, exclusion criteria), brain imaging methods (e.g., CT vs. MR imaging) and measurement techniques (e.g., linear vs. volume measures), and approach to statistical analysis, including adjustments of cognitive test data for potential confounding variables. The negative findings from these studies
4,5,25,26 may also have been the result of low power due to relatively small sample sizes.
Memory. We found that lateral ventricle volume and third ventricular volume were each associated with poorer recall on visual delayed memory (
Table 5). Our results stand in contrast to negative findings from the three previous studies that have examined relationships between ventricular size and visual memory (
Table 1).
5,24,26 As discussed above, these negative findings are difficult to compare with the findings in the present study because of methodological differences, and they also suffer from low power associated with relatively small sample sizes. More recently, visual explicit memory (variously measured) has also been linked to the size of the visual cortex
20 and corpus callosum.
21 Conflicting findings have been reported for the hippocampus (
Table 1).
6,8,13,23 We found no relationship between verbal delayed memory and either age-specific cerebral atrophy (either cerebral hemisphere volume or peripheral CSF volume) or ventricular enlargement, a result that is in agreement with most of the extant literature (
Table 1).
5,7,10,20,24–26 In contrast, Carmelli et al.
4 observed that the difference in brain volume between elderly male identical twins was associated with within-pair differences in memory performance on the California Verbal Learning Test (
Table 1). Several other studies have examined relationships between verbal memory and discrete structures such as the medial temporal lobe, with conflicting findings reported for hippocampal size
6–8,13,15,20,23 and negative results observed for the amygdala
23 and parahippocampus (
Table 1).
13,20Language and Visuospatial Function. We found no relationship between our two measures of language function (Fluency, Boston Naming) and either cerebral atrophy (cerebral hemisphere volume or peripheral CSF volume) or ventricular enlargement (
Table 5). Others (
Table 1) have likewise reported no relationship between CSF volume and various measures of language function,
25 including the Boston Naming Test.
16 In contrast, Stafford et al.
24 found that total CSF volume on CT was related to a discriminant function of naming and abstraction that included the Boston Naming Test. O'Brien et al.
15 recently reported that language function was not associated with age-related atrophy of the amygdala-hippocampal complex.
We also found no relationship between our measure of visuospatial construction (Block Design) and either cerebral atrophy (cerebral hemisphere volume or peripheral CSF volume) or ventricular enlargement (
Table 5). All published studies of which we are aware have likewise reported no relationship between performance on the Block Design test and age-related changes in peripheral CSF volume
25,26 or ventricular size (
Table 1).
5,25,26 Performance on other visuospatial tasks has likewise shown no relationship to age-related lateral ventricular enlargement.
14 No previous study has examined relationships between Block Design performance and age-related changes in cerebral hemisphere volume per se, although the former was found to be unrelated to the size of corpus callosum, pons, or cerebellum (
Table 1).
21In summary, our data suggest that global age-related changes in brain size, both cortical (e.g., cerebral atrophy and increased peripheral CSF volume) and subcortical (e.g., ventricular enlargement), are associated with age-related decrements in attention, psychomotor speed, and visual delayed memory. These results are consistent with other data suggesting that the neural substrates for these functions involve multiple systems distributed throughout the brain. Still other functions, such as verbal memory, language, and visuospatial functioning, were not related to age-linked changes in cerebral size in our study. Attempts by others to relate these functions to more discrete brain structures have met with inconsistent results (
Table 1). These discrepant findings regarding relations between cognition and brain structure might mean that cognitive aging is truly a function of diffuse global brain changes, or that age-related cognitive decline is multifactorial, such that changes in several brain regions may have similar effects on cognitive test performance. Slowing on Trails B, for example, could be caused by reduced motor speed, impaired working memory, poor visual scanning, or several cognitive deficits. A finer-grained analysis of the relationships between the specific changes associated with aging and the morphology of more discrete brain structures is required to resolve this issue.