Demographic Findings
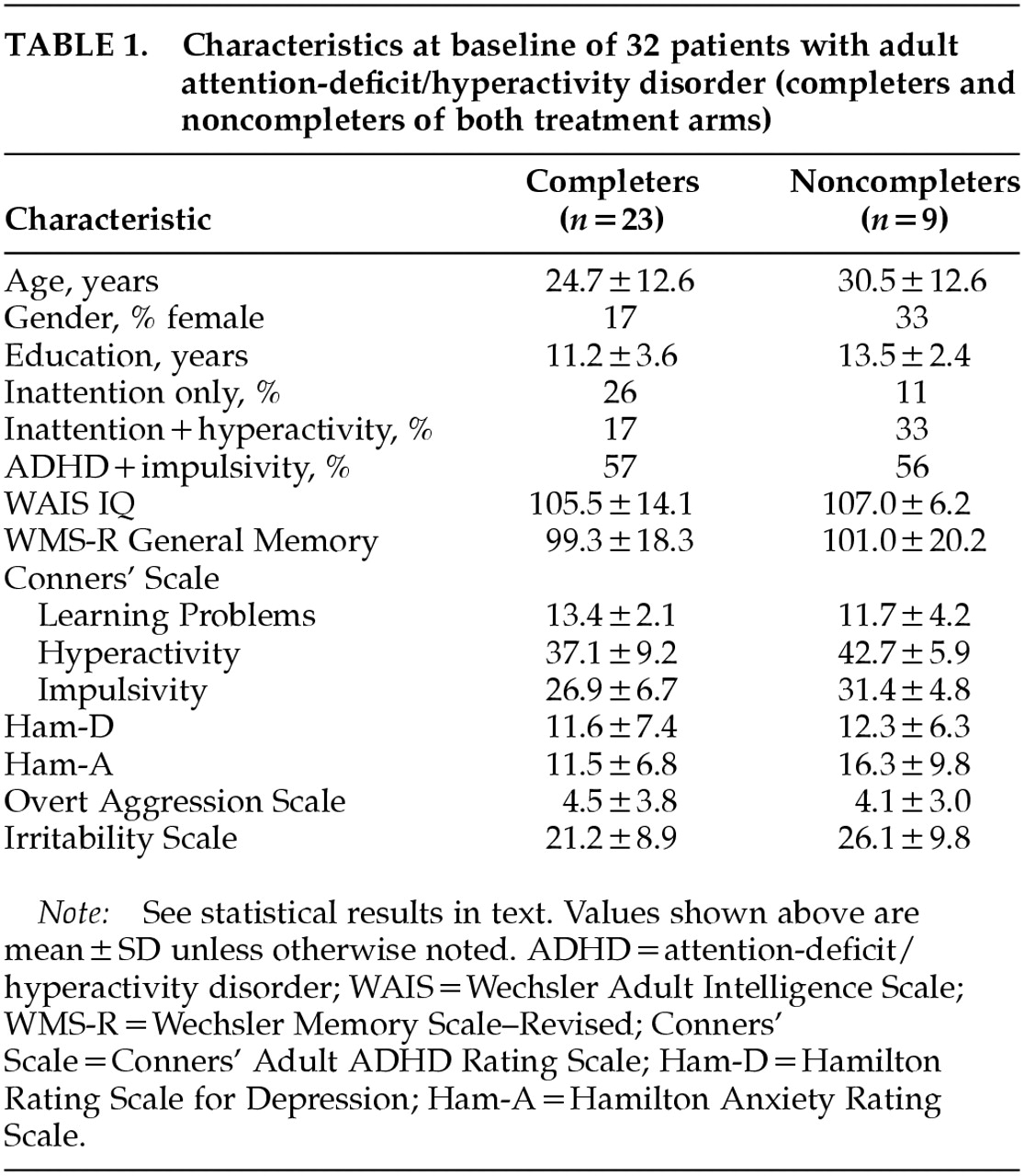
Four patients (2 on lithium and 2 on MPH) did not complete the first and second treatment arms, and 5 patients (3 on MPH and 2 on lithium) did not complete the second arm. Twenty-three patients completed both arms. They were 19 men and 4 women, with a mean age of 24.7±12.6 years (range 18–60), a mean education level of 11.2±3.6 years (range 7–19), a WAIS Full Scale IQ score of 105±14.1 (range 81–130), and a WMS-R Memory Quotient score of 99.3±18.3 (range 67–131). Twenty-two percent of the sample met DSM-IV criteria for inattention only; 15% had hyperactivity and impulsivity without inattention; and the remaining 63% had the full syndrome.
Table 1 shows background information for both completers and noncompleters. There were no significant differences in age, education, full scale IQ, or Ham-D, Ham-A, or OAS scores between completers and dropouts. On the other hand, dropouts had significantly higher Conners' Adult ADHD Rating Scale scores in the clusters of Conduct Disorder (
P<0.05), and Hyperactivity (
P<0.05) compared with completers.
Eleven of the 32 patients (34%) had at least one lifetime comorbid psychiatric disorder; 8 had a major depressive syndrome, 2 had social phobia, and 1 had a history of panic attacks. Twenty of the 32 patients (63%) had a positive history of ADHD in first-degree relatives. Despite average intelligence, school problems were reported by 20 of the 32 patients (63%); 14 patients (44%) failed at least one grade, 2 (6%) were placed in special classes, and 4 (13%) were expelled from school. A history of serious working difficulties (e.g., frequent job changes or disciplinary measures) was positive for 80% of the sample. This information was always checked with parents and/or siblings.
Primary Efficacy Measures
Average daily doses of MPH (mg) for the 23 completers were the following: weeks 1–2, 10±0.0; weeks 3–4, 19.5±1.4; weeks 5–6, 38.9±5.2; and weeks 7–8, 38.9±5.2. Average daily doses of lithium carbonate (mg) and corresponding plasma lithium levels (mEq) were the following: weeks 1–2, 600±0.0 and 0.43±0.15, respectively; weeks 3–4, 886±62.5 and 0.50±0.18; weeks 5–6, 1,186±62.5 and 0.65±0.21; and weeks 7–8, 1,173±125 mg and 0.68±0.25. Final doses for both drugs did not match planned doses because some patients could not tolerate the targeted final dose.
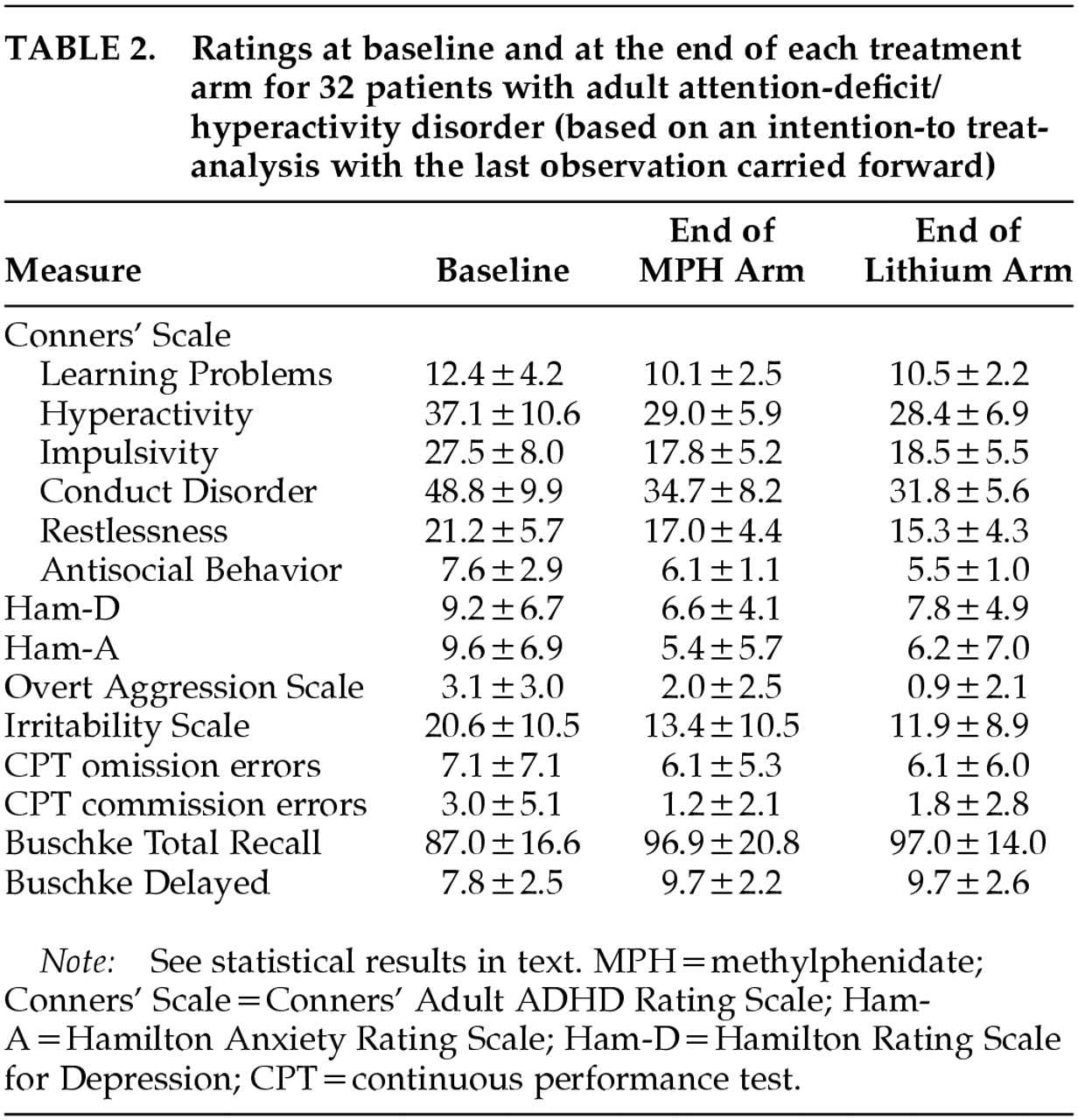
Mean ratings on all measures at baseline and at the end of each treatment arm are displayed in
Table 2. Changes over time in Conners' Adult ADHD Rating Scale cluster scores are shown in
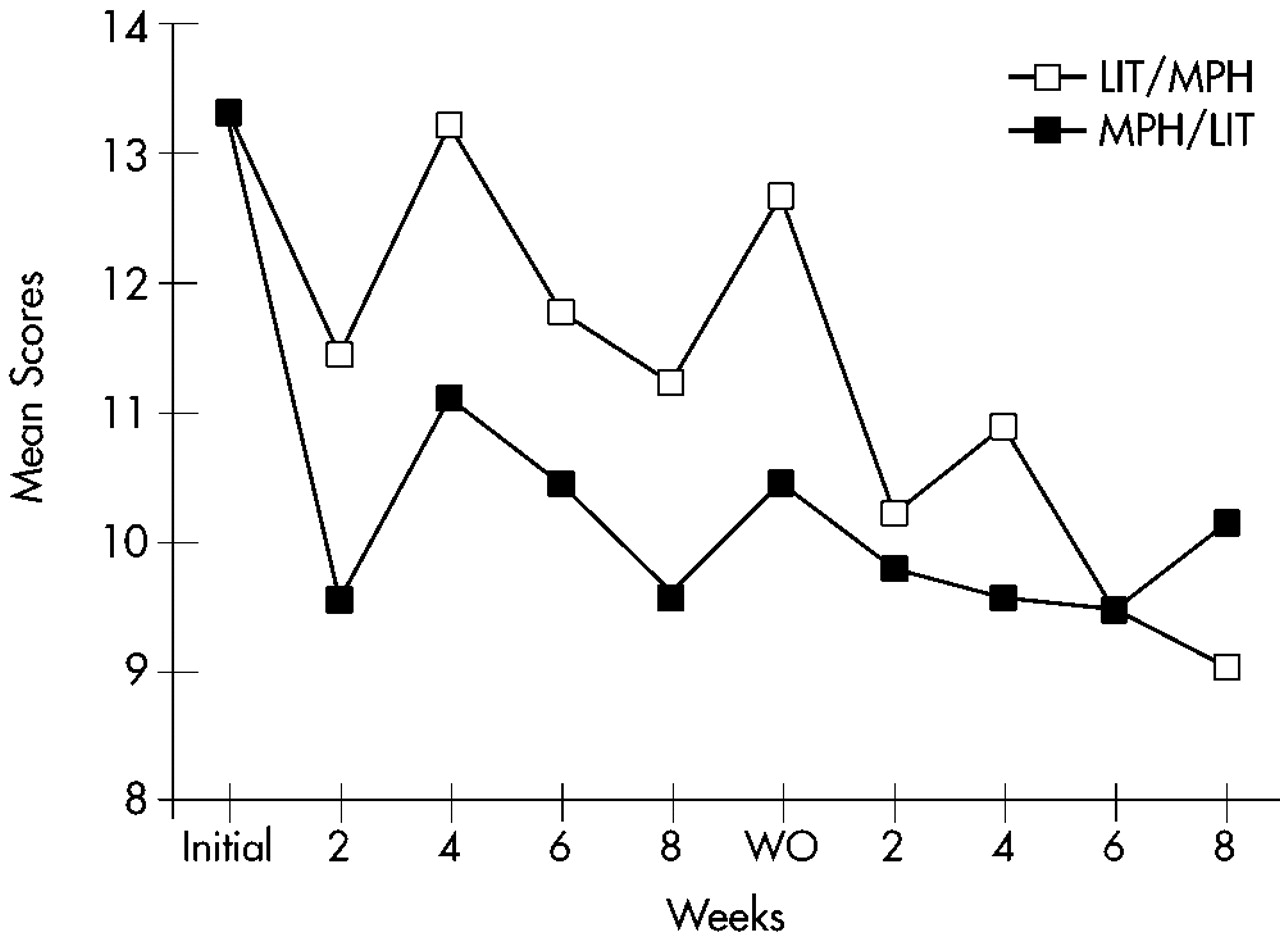
Figure 1 for the Learning Problems cluster,
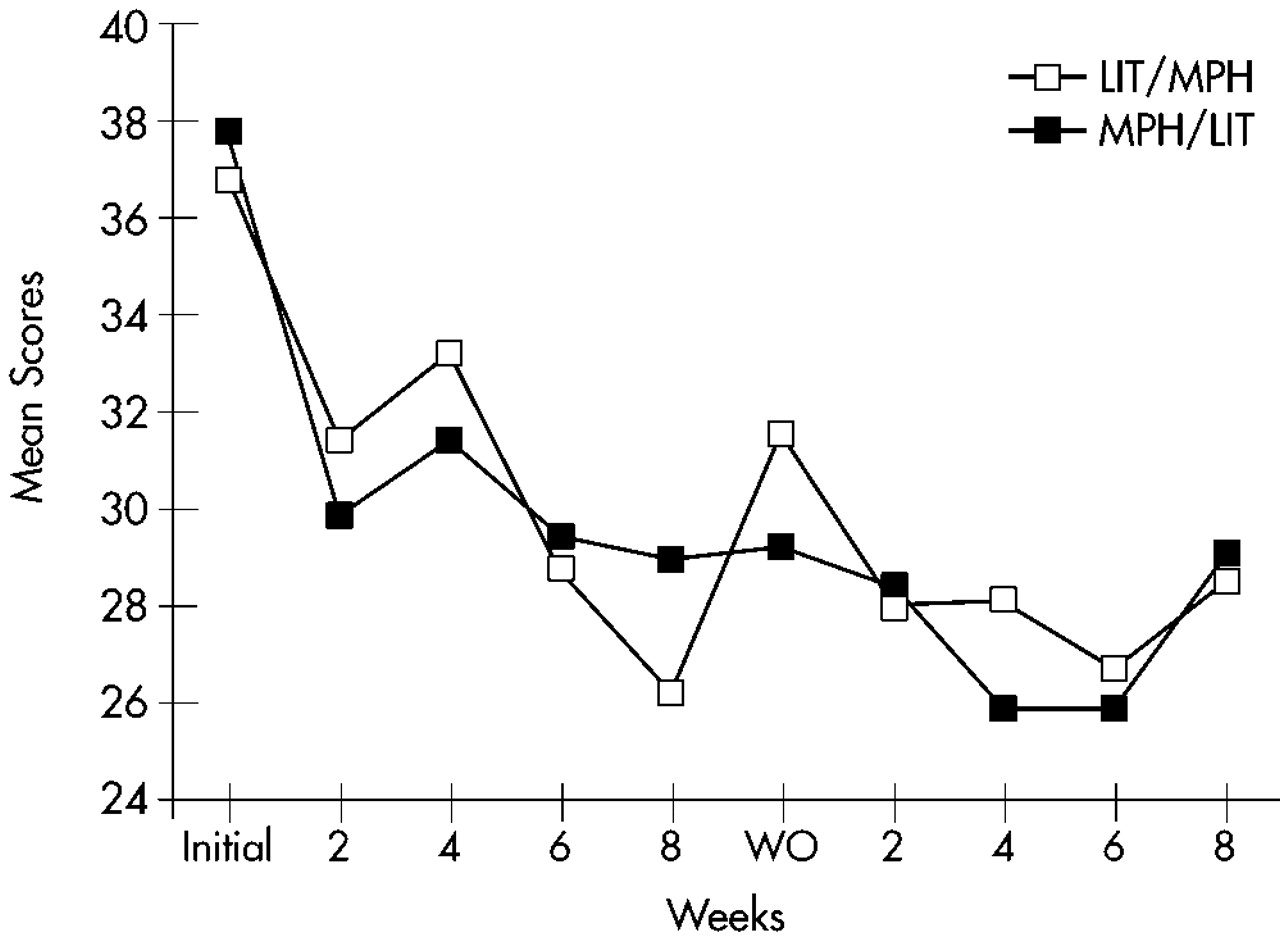
Figure 2 for the Hyperactivity cluster, and
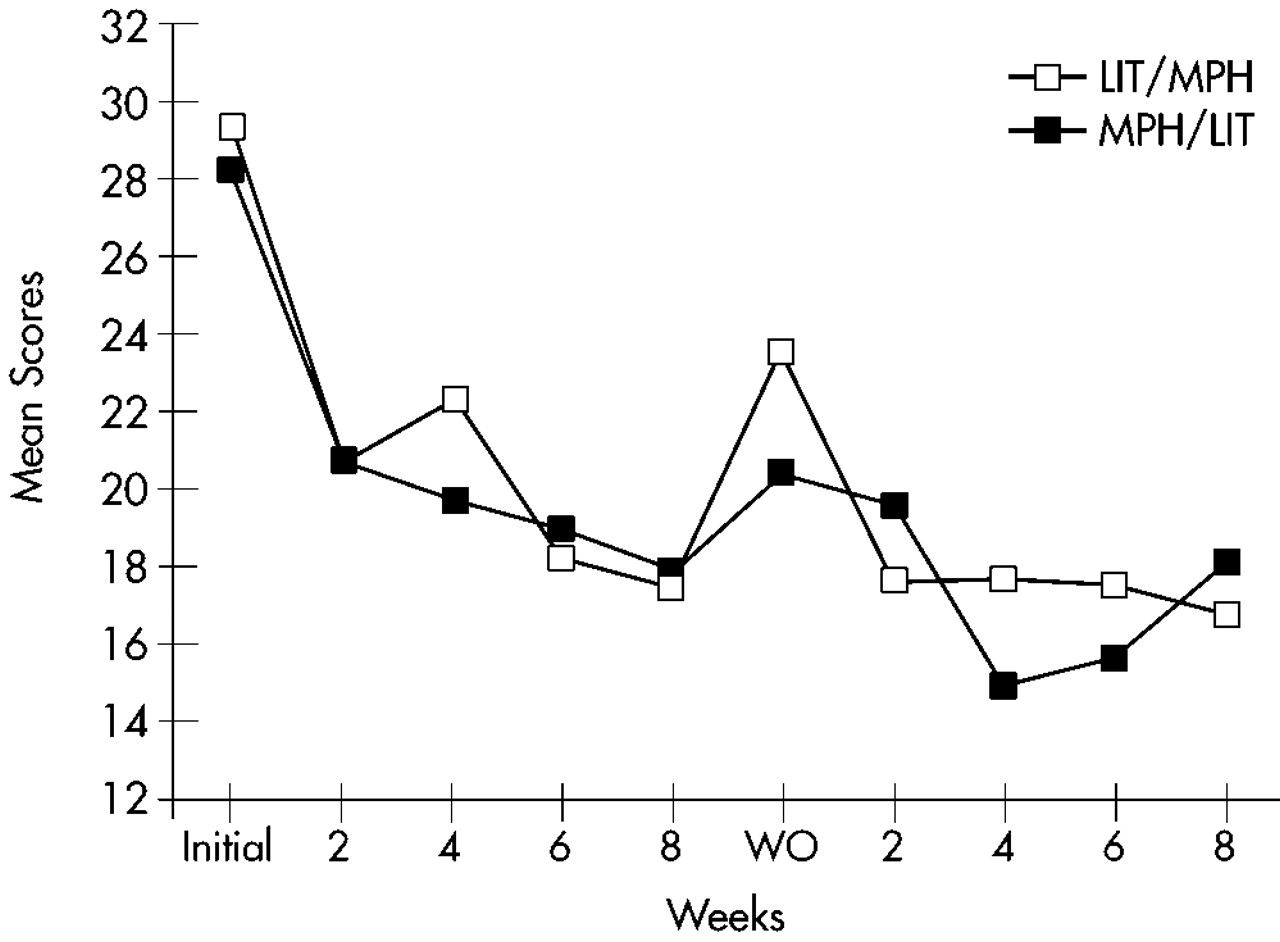
Figure 3 for the Impulsivity cluster.
The rate of improvement, defined as 30% or greater reduction in the Conners' Adult ADHD Rating Scale sum score of Learning Problems, Hyperactivity, and Impulsivity compared with the first baseline evaluation, was 48% for MPH and 37% for lithium (95% confidence interval [CI], –12% to 34% for the observed difference between MPH and lithium). For completers, the rate of improvement was 47% for MPH and 43% for lithium (95% CI, –23% to 31% for the observed difference between MPH and lithium). Three of the 10 patients who were nonresponders to lithium during the first arm did respond to MPH during the second arm, but none of the 9 nonresponders to MPH during the first arm had a positive response to lithium during the second arm. There were no significant differences in lithium serum levels between lithium responders and nonresponders (mEq/l [mean±SD]=0.69±0.39 and 0.65±0.20, respectively).
A three-way repeated-measures ANOVA (Factor 1: sequence [MPH/lithium vs. lithium/MPH]; Repeated measures: arm [first vs. second] and time [5 evaluations]) for all 32 patients randomized into the study (ITT analysis with LOCF) was calculated for each of the three primary outcome measures. The Hyperactivity cluster of the Conners' Adult ADHD Rating Scale showed a significant arm effect (F=15.3, df=1,28, P<0.0001), with a significantly greater improvement during the first compared with the second arm; a significant time effect (F=13.1, df=4,112, P<0.0001), with a significant improvement over time for both arms; no significant effects for sequence (F=1.06, df=1,28, not significant) or sequence×arm interaction (F=0.32, df=1,28, not significant), with MPH and lithium showing similar efficacy; and no significant sequence×arm×time interaction (F=1,75, df=4,112, not significant), with MPH and lithium showing similar magnitudes of improvement over time. Similar results were found for the Impulsivity and Learning Disorders clusters of the Conners' Adult ADHD Rating Scale (Impulsivity: arm effect, F=6.83, df=1,28, P<0.05; time effect, F=22.4, df=4,112, P<0.0001; Learning Disorders: arm effect, F=9.72, df=1,28, P<0.01; time effect, F=13.1, df=4,112, P<0.01). The remaining main effects and interactions were not significant.
Hyperactivity and Impulsivity scores at the baseline evaluation for the second arm were significantly lower than scores for the initial baseline evaluation (Hyperactivity: first baseline score [mean±SD]=36.7±8.7, and second baseline score=31.6±7.7; t=3.23, df=28, P<0.001; Impulsivity: 27.4±6.8 and 22.7±6.7, respectively; t=3.71, df=28, P<0.001). There were no significant changes in scores of Learning Problems and Hyperactivity between the last evaluation of the first arm and the baseline evaluation for the second arm (Learning Problems: t=1.47, df=28, not significant; Hyperactivity: t=1.32, df=28, not significant), but scores of Impulsivity had a significant increment during this interval (t=2.57, df=28, P<0.05).
To examine whether AD symptoms reemerged during the washout period, we compared scores on the ADHD clusters for the baseline and the washout evaluations. Patients on MPH had significantly lower scores on the washout as compared to the baseline evaluation for the clusters of Learning Disorders (t=2.28, df=9, P<0.05), Hyperactivity (t=5.93, df=9, P<0.001), and Impulsivity (t=7.91, df=9, P<0.0001). Patients on lithium had significantly lower scores on the washout compared with the baseline evaluation for the cluster of Impulsivity (t=2.83, df=9, P<0.05), but no differences were found for the clusters of Learning Disorders (t=0.43, df=9, not significant) and Hyperactivity (t=1.87, df=9, not significant).
Secondary Efficacy Measures
The Conduct Disorder cluster of the Conners' Adult ADHD Rating Scale showed a significant arm effect (F=11.7, df=1,28, P<0.01), and a significant time effect (F=27.9, df=4,112, P<0.0001), and there were similar results for the Restlessness (arm, F=11.1, df=1,28, P<0.01; time, F=14.0, df=4,112, P<0.0001) and Antisocial Behavior clusters (arm, F=17.4, df=1,28, P<0.0001; time, F=12.1, df=4,112, P<0.0001).
Statistical analysis of the Irritability scale demonstrated significant arm (F=5.47, df=1,28, P<0.05) and time effects (F=12.0, df=4,112, P<0.0001), and similar results were found for the Ham-A (arm, F=5.88, df=1,28, P<0.05; time, F=11.7, df=4,112, P<0.0001). There was only a time effect for both the Ham-D (F=5.45, df=4,112, P<0.001) and the Overt Aggression Scale (F=5.73, df=4,112, P<0.001). No other significant main effects or interactions were found.