Borderline personality disorder (BPD) patients constitute a large burden on the resources of mental health services. Developing a thorough understanding of the neurobiology of BPD is essential for the development of effective preventive, therapeutic, and rehabilitative approaches to BPD. Although BPD is one of the most investigated of the personality disorders, the neurobiological basis of this devastating disorder remains largely unknown. Evidence for an organic basis for BPD has been forthcoming since the 1980s.
1,2 Efforts to bring rapidly advancing neuroinvestigative technology to bear on the understanding of this disorder is likely to contribute significantly to the unraveling of the underlying pathophysiological processes and the identification of any biological subtypes this disorder might have.
Electrophysiological technology is both noninvasive and relatively inexpensive. Every major university has an electrophysiology research laboratory. We performed this literature review in order to encourage and facilitate the involvement of these laboratories in the investigation of the neurobiology of BPD. Thus, in our examination of the literature, we had two goals in mind: first, to assess whether or not electrophysiological lines of investigations are likely to be profitable, and second, to highlight difficulties and shortcomings in the research in order to guide future research efforts.
A number of electrophysiological studies linked BPD to complex partial seizures (CPSs).
3 Andrulonis et al. found that 27% of adolescent BPD patients had evidence of brain dysfunction or current epilepsy.
4 They also found history of head trauma, encephalitis, or past seizures in 11%. Several episodic or paroxysmal symptoms are common between BPD and temporal lobe epilepsy: impulsivity, transient psychosis, and intermittent experience of depersonalization and derealization.
5 Carbamazepine has been shown to be effective in decreasing paroxysmal symptoms.
6 Indeed, a number of case reports have described CPSs in patients who had previously been diagnosed with BPD.
7–10In a separate set of reports, BPD has been linked to affective disorders.
11 A high rate of co-occurring depressive symptoms among BPD patients has long been observed. Indeed, longitudinal studies have found that even cases of apparently pure BPD, when followed over time, are characterized by frequent suicide attempts and clear-cut affective episodes.
12 Steinberg et al. found an exaggerated depressive response to physostigmine challenge in BPD patients as compared with patients with other personality disorders.
13In a third branch of the literature, BPD has been linked to psychotic disorders.
14,15 The partial clinical response among BPD patients to neuroleptics further supports a possible relationship to psychotic disorders.
16,17 Early outcome studies have generally found that the long-term adjustment of patients with BPD is similar to that of patients with schizophrenia.
14,18 More recent follow-up studies of these patients showed that their long-term outcome is comparable with that of patients with unipolar depression and overall more favorable than that of patients with schizophrenia.
19,20In a review of the available literature a decade ago, Korzekwa et al. suggested that BPD is an independent disorder with at least two possible biological subtypes: an affective subtype and a psychotic subtype.
21 Coccaro and Kavoussi suggested that the identification of such subtypes can be useful in guiding treatment choices.
22A better understanding of electrophysiological abnormalities in BPD will also facilitate the examination of the relationship between abnormalities related to child abuse and those identified in adult patients. Child abuse has been shown to result in clinical electroencephalogram (EEG),
23 evoked potentials (EPs), as well as quantified EEG and EEG coherence abnormalities.
24,25 Similarly, child abuse has been shown to adversely affect sleep.
26 Child abuse has been strongly linked to the development of BPD.
27–29 Electrophysiological abnormalities were also reported in association with other stress-related disorders such as posttraumatic stress disorder.
30,31 A more complete characterization of such abnormalities may also shed more light on the interrelationships between these disorders.
Electrophysiological technology has rapidly advanced in the past decade. Computerized quantification of electroencephalography with the use of high-density electrode arrays has made it capable of accurate localization of the cerebral sources of scalp-recorded activity. The technology for recording and analyzing sleep (polysomnography) and cerebral EPs has similarly advanced, allowing more detailed examination of these biological signals. Moreover, newer, powerful techniques such as magnetoencephalography, which is capable of examining the EEG signal of deeper cortical tissue, and transcranial magnetic stimulation, capable of noninvasively examining the excitability characteristics of the cortex in awake, behaving humans, have yet to be applied to the investigation of BPD.
In order to assess the value and possible future contribution of electrophysiological investigative techniques, we examined the literature in which at least one of these techniques was used to examine patients with BPD.
METHOD
Exhaustive MEDLINE and PsycInfo searches were performed for the period of 1966 to 2000 for all articles cross-referenced for “biological aspects” and “BPD.” Additional searches were conducted using the terms EEG, evoked potentials (or EP), sleep, and polysomnography (or PSG). Articles referenced in these articles that had an electrophysiology component were also reviewed, and review articles were used to identify additional original research articles. For inclusion in our review, papers had to be in English and had to report studies that included a group of BPD subjects (with or without other groups) and used at least one electrophysiological testing technique. Single case reports were also included, as they have heuristic value to this line of investigation. All articles were independently reviewed in detail by two of the authors (M.T. and N.N.B.).
The two reviewers extracted the following information from each article: the method used for clinical evaluation, particularly the diagnostic criteria applied; the presence or absence of comorbid conditions (both Axis I and Axis II); medications used by subjects at time of study; the number and gender of the study subjects; and the electrophysiology modality used.
RESULTS
We found two review articles addressing the contribution of the different electrophysiological modalities to the study of BPD.
2,21 Lahmeyer et al.
2 concluded that sleep in general and rapid eye movement (REM) sleep in particular are abnormal in BPD patients as a group. They further suggested that several factors, including concurrent Axis I affective psychopathology, family psychopathology, and a personal past history of depression predict REM sleep abnormalities, particularly shortness of REM latency, in this group. On the basis of the limited number of evoked potentials available at the time, they concluded that a link between BPD and psychosis is likely to exist. Korzekwa et al.
21 concurred with Lahmeyer et al., noting that BPD patients have abnormal REM sleep but that the abnormality is most pronounced when depression coexists with BPD. They also agreed with Lahmeyer et al.'s conclusion that evoked potential studies suggest a link between BPD and schizophrenia. However, neither of these articles specifically focused on electrophysiological measures, examined the composition of the groups studied, reported on sample sizes, comorbidity, medications, or diagnostic systems used, or included reviews of clinical EEG data in this population.
A total of 24 original research articles were selected for our review. Nine of the articles (among them two case reports) used standard EEG, five used EPs, eight used sleep or polysomnography, and two used computer-based EEG spectral analysis.
Standard EEG Studies
Standard EEG studies (
Table 1) have been carried out under the hypothesis that abnormal brain electrical activity and/or focal brain dysfunction, particularly in the temporal lobes, play a significant role in the pathogenesis of BPD characterized by impulsiveness and affective instability. A number of case reports described patients who were diagnosed with BPD who were subsequently found to have complex partial seizures documented by epileptic discharges over one or both temporal regions
6 and favorable clinical response to anticonvulsant medications.
10 By the mid-1980s, the presence of significant EEG abnormalities in BPD patients was well documented. Snyder and Pitts showed that patients with BPD have a significantly higher rate of both definitive and less definitive EEG abnormalities compared with a group with dysthymic disorder.
7 In this report, older BPD patients had more severe EEG abnormalities. Abnormalities (mainly slowing) were most frequently bilateral and of frontal, temporal, or frontotemporal distribution. Similarly, Cowdry et al. examined symptoms and EEG changes in 39 BPD patients.
8 BPD patients showed a much higher incidence of symptoms commonly seen in complex partial seizures or episodic dyscontrol than a control group of unipolar depressed patients. BPD patients also showed a much higher incidence of paroxysmal EEG activity, particularly posterior sharp waves. Archer et al. found that 6.3% of a group of 16 adolescents with BPD had bilateral spike and wave discharges, whereas none of 10 subjects in a comparison group with other personality disorders had similar discharges.
32 In general, a higher incidence of severe abnormalities was found in BPD as compared with other personality disorders.
33 Furthermore, minor abnormalities that are not suggestive of epilepsy but may contribute to episodic behavior (e.g., 14 and 6 positive spikes) were found in 25% of BPD patients, 30% of patients with other personality disorders, and 18% of dysthymic patients.
Ogiso et al.
34 provided evidence of different EEG correlates of BPD symptom profiles. Using subscales of the Diagnostic Interview for Borderline Personality Disorder, they found the presence of positive spikes to correlate with impulsivity and the presence of spike and wave phantoms to be correlated with high scores on interpersonal relationship dysfunction. In the mid-1980s, the phantom spike and wave discharges were correlated with the presence of neurovegetative symptoms (e.g., headaches and body aches).
35 More recently, positive spikes were found to be a correlate of attention deficit disorder.
36 Ogiso et al. emphasized the observation that no one pattern characterized the entire sample and that in some patients the EEG was perfectly normal.
34 Similarly, Drake et al.
37 found that BPD patients who presented with psychogenic seizures had normal EEGs.
De la Fuente et al.
38 reported a 40% incidence of diffuse EEG slowing in a group of unmedicated BPD patients. None of their patients received neuroleptic drugs for at least 2 months, and all other medications were withdrawn for 10 days, or 15 days for tricyclic antidepressants and monoamine oxidase inhibitors. The presence of active Axis I comorbid disorders, including current drug abuse (as verified with repeated plasma testing), was ruled out. Additionally, any history of neurological problems, including seizures, was grounds for excluding subjects. Carbamazepine did not appear to modify the EEGs of the patients in this sample. The finding of a high prevalence of slow wave abnormalities in BPD patients is in support of an earlier report by Tanahashi.
39 Based on the English abstract of the Japanese-language paper, Tanahashi compared EEG findings between patients with BPD and patients with schizophrenia.
39 He found a significantly higher incidence of slowing of background activity among the BPD group (84.4% of BPD patients vs. 32% of schizophrenia patients). He also reported spike and wave complexes (of the 6/second variety) in 31% of the BPD patients, compared with 4% of the schizophrenia patients. We have previously reported that the presence of diffuse EEG slowing is correlated with the overall severity of psychopathology.
40In summary, only two studies reported controlling for Axis I or Axis II comorbidity. It is not clear from the papers how carefully these evaluations were conducted. No mention was made in any of the papers of the level of training of the evaluators. Only two of the studies included control groups with non-BPD personality disorders. Control for medication effects was reported in four of the seven papers reviewed (excluding the two case reports
9,10). In its current state, this literature suggests that two types of standard EEG abnormalities may exist in this group of patients. The first is the presence of epileptiform discharges. This type of abnormality is likely to indicate decreased threshold for seizure-like activity or increased cortical excitability and may be predictive of responsiveness to anticonvulsant therapy.
41The second type of standard EEG abnormality is the presence of diffuse EEG slowing. The presence of diffuse slowing in unmedicated subjects indicates either a metabolic or a degenerative brain disorder. Patients with mental retardation could also exhibit diffuse slowing of the EEG. The presence of this abnormality should lead to further workup of the patient to identify causes of encephalopathy. The presence of a static (nonprogressive) and nonmetabolic diffuse EEG slowing could indicate that the patient is in a more difficult group of patients who are less likely to respond to pharmacotherapy.
40 Early EEG studies have underscored the fact that the clinical correlates of EEG abnormalities in BPD is unlike the more straightforward correlations that can be seen in epilepsy patients and that factor analyses of symptom clusters are necessary to examine such correlates.
32,33Evoked Potentials
A second set of studies examined the cerebral evoked potentials (EPs) (
Table 2) in an effort to probe the possible relatedness of BPD and schizophrenia
42 and schizophrenia spectrum disorders.
43 Abnormalities in EP correlates of information processing in BPD patients were first reported by Blackwood et al.,
44 who showed that the amplitude of the P300 EP (a positive wave occurring 300 msec after detection of a novel or unexpected stimulus) was smaller and that its latency was delayed in comparison with a group of normal control subjects and a group of patients with non-BPD personality disorders. Kutcher et al.
45 showed that the presence of a low amplitude or a prolonged latency of the P300 EP is common only in BPD, schizotypal personality disorder, and schizophrenia patients. The presence of this abnormality differentiated the BPD patients from normal control subjects, patients with depressive disorders, and patients with personality disorders other than schizotypal personality disorder. Moreover, Kutcher et al. found no effect of psychotropic medications on P300 in their subjects. In an earlier study using both normal and patient control groups, the same researchers showed that P300 changes distinguished the BPD patients and schizophrenia patients from normal control subjects, patients with major depression, and patients with non-BPD personality disorders.
46While the literature on EPs in BPD is limited, the finding of evidence of abnormal information processing is of interest. Drake et al.
47 provided evidence that EP abnormalities in BPD patients may be more extensive than what Kutcher et al. reported.
46 They found both mid-latency and long-latency EPs to have prolonged latencies and decreased amplitudes. These abnormalities are very similar to EP abnormalities reported in patients with schizophrenia.
48 Lincoln et al.,
49 investigating EPs in children with BPD, found abnormalities similar to those seen in adult patients, particularly in the auditory modality. EP changes are commonly seen in association with psychotic states, including schizophrenia spectrum disorders (e.g., schizotypal personality disorder) and less frequently with mood and anxiety disorders. The widely reported EP abnormalities in psychotic states are the low amplitude and prolonged latency of the P300 EP component.
48 The presence of such abnormalities in BPD patients may be indicative of a psychotic process and could be predictive of a favorable response to antipsychotic medications.
The literature on EP investigations of BPD is limited. Of the five studies included in this review, only three reported controlling for comorbidity. As in the EEG literature, none reported the qualifications of the evaluators or how closely the comorbid disorders were assessed. Non-BPD Axis II control groups, particularly with schizotypal personality disorder, were included in three of the five studies. As with EEG studies, attention was paid to the compounding effects of medications.
Sleep Studies
The study of sleep architecture using polysomnographic evaluations has a long and extensive history in the probing of affective disorders. Rapid eye movement measurements (i.e., latency and density) emerged as likely indicators of the liability of developing affective disorders.
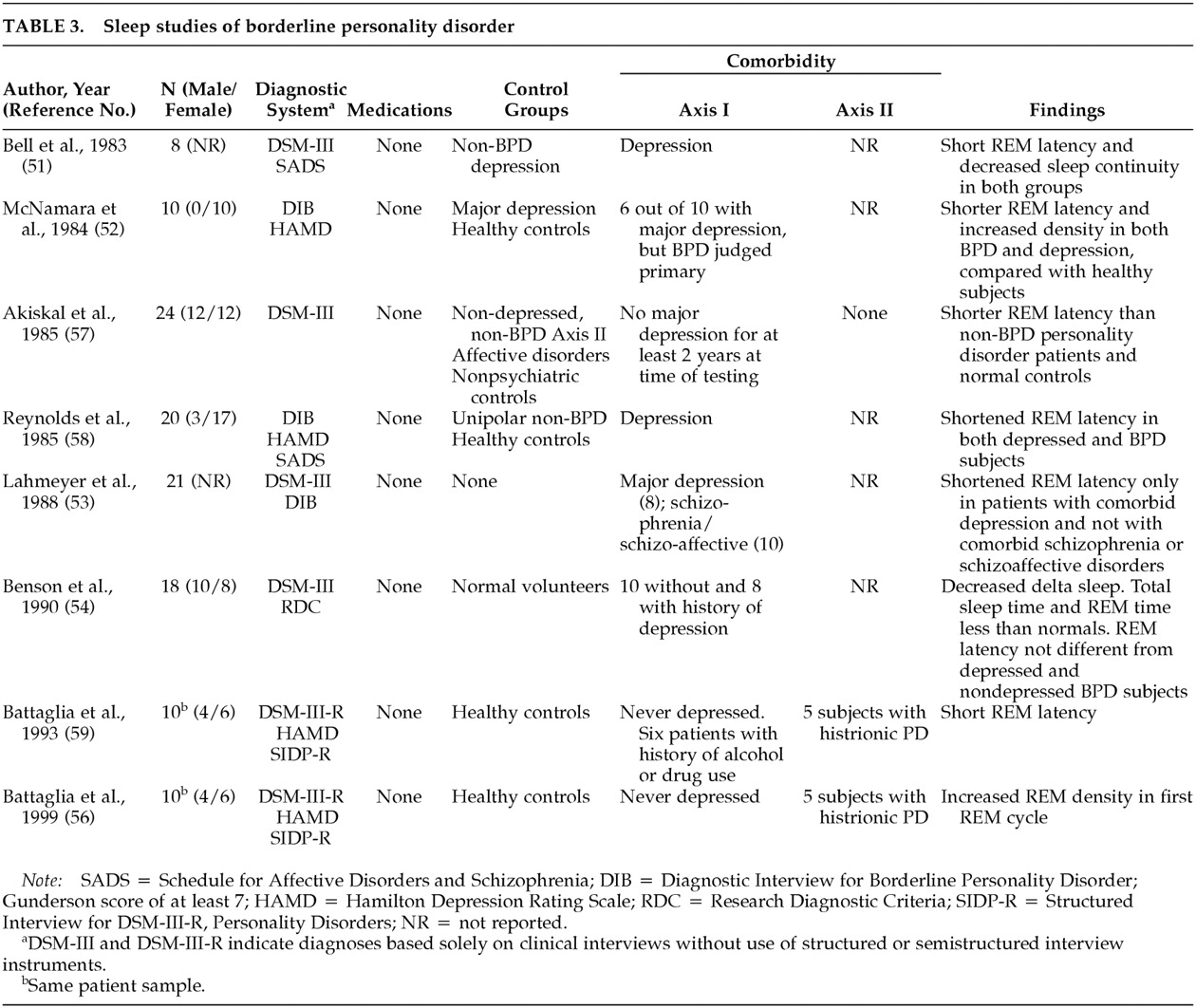
50 Driven by the hypothesis that BPD may be an affective disorder or that a subgroup of BPD patients may have a significant affective component to their illness, sleep architecture was extensively examined in BPD (
Table 3).
Since the early 1980s, sleep studies have shown that, as a group, patients with BPD have abnormal sleep patterns. Depressed BPD patients had REM sleep changes similar to those of depressed patients without a personality disorder.
51 Shortened REM sleep latency in patients with BPD was more variable than in depressed patients and needed at least 2 nights of recording to be clearly differentiated from that of normal control subjects.
52Lahmeyer et al.
53 examined the correlations between clinical symptom clusters and sleep changes in 21 BPD patients. They found that patients with comorbid diagnoses of schizophrenia, schizoaffective disorder, or mania had normal sleep architecture. In contrast, BPD patients with comorbid depression tended to have short REM latency and high REM density. Benson et al.
54 found REM sleep onset to be shorter in BPD patients who were not depressed (100 ± 44 minutes for controls and 82.9 ± 29 for BPD patients), although the difference was not statistically significant. They also provided data that other sleep measures (e.g., sleep continuity, total sleep time, and percentage of stage 4 sleep) were abnormal. These changes are similar to sleep changes reported in schizophrenia patients
55 and suggest that sleep changes in BPD may not be limited to REM stage parameters.
A more recent report found REM sleep density to be elevated in BPD patients who had never been clinically depressed according to DSM criteria.
56 Compared with nondepressed patients with non-BPD personality disorders who did not meet criteria for schizotypal personality disorder, nondepressed patients with BPD had significantly shorter REM latencies.
57 REM latency and density were found not to differ between groups of nondepressed BPD patients, depressed BPD patients, and depressed patients without an AXIS II disorder.
58Battaglia et al.
59 used ambulatory EEG monitoring to avoid the effects of the laboratory on sleep measurements. They found REM latency to be significantly shorter in BPD patients than in a normal control group. They did not find an increase in REM density in their BPD sample.
As
Table 3 shows, the most frequently reported sleep architectural change in BPD patients is shortening of REM latency. This abnormality is most frequently reported in association with mood disorders. The detection of REM sleep changes may be indicative of an underlying mood disorder and could predict a favorable response to antidepressant therapy. Although sleep architecture is the most extensively examined electrophysiological aberration in BPD, this literature remains limited and exhibits the same shortcomings noted for the EEG and EP studies. The emphasis in this literature is on either controlling for or excluding depressive disorders; four of the seven studies excluded depressive disorders in BPD subjects. Five of the studies did not include non-BPD Axis II control groups.
Other Physiological Measures
A number of other physiological measures were applied to the examination of BPD. The literature on these measures is extremely limited, but the data generated are of interest, and these studies were included here for the sake of completeness. Using EEG spectral analysis, Cornelius et al.
60 found no correlation between EEG spectra and depressive or transient psychotic symptoms. On the other hand, they found that the mean frequency values were consistently correlated with anxiety levels. Using power spectral technology, Russ et al.
61 found that theta activity was significantly correlated with pain ratings in BPD patients with and without self-injurious behavior. Power spectral and high-density EEG technology allows the examination of more subtle EEG changes than standard EEG allows. Verkes and colleagues
62 examined the circadian rhythm in 59 patients with recurrent suicidal attempts. They found suicidal ideations, BPD, and impulsiveness to be associated with the absence of a clear 24-hour periodicity in motor activity. Finally, Siever et al.
43 found that 10 of 13 BPD patients had abnormal smooth-pursuit eye tracking. Eye tracking dysfunction is prevalent in psychotic disorders and has been proposed as an endophenotypic marker of schizophrenia.
63DISCUSSION
Two conclusions can be reached on the basis of our review. The first is that electrophysiological investigation of BPD is limited. In its current state, the literature does not allow the development of specific hypotheses about etiology, pathophysiology, or phenotypic expressions. This conclusion is not unexpected given the small number of studies, the complexity (and possible heterogeneity) of the disorder, the complex problem comorbidity on both Axis I and Axis II, the evolving diagnostic criteria, and the confounding effects of pharmacotherapy. Moreover, no longitudinal studies have been attempted thus far. Such studies are essential in differentiating state- from trait-dependent changes and in documenting the effects of pharmacotherapy on physiological parameters in this patient population. Longitudinal studies may be particularly revealing given that physiological aberrations identified in adult BPD patients may well have their origin in childhood trauma.
Studies assessing the prognostic value of physiological changes are similarly lacking. Electrophysiological investigations of BPD have the potential to contribute to our understanding of the different pathophysiological processes that may be aberrant in BPD patients. This is suggested by the findings in the reviewed studies. Specifically, standard EEG studies can be useful in probing the relationship of BPD to complex partial seizures, sleep studies can help probe the relationship of BPD to mood disorders, and EP studies can help elucidate the commonalities between BPD and psychotic disorders.
In only one of the studies we examined was more than one modality applied in the same subjects.
53 Findings from this study suggest that the abnormalities discussed above may not coexist in the same subjects and may be indicative of different subtypes. In addition, in none of the studies reviewed has the entire set of electrophysiological measures been recorded in the same set of patients. Curiously, over the past several years, far fewer brain electrophysiological studies of patients with BPD have appeared, although during that period electrophysiological recording technology has improved significantly. We now have the ability to study various components of brain activity from a large number of scalp locations simultaneously. This should improve the capacity to reliably detect brain abnormalities in BPD patients. A comprehensive examination of the electrophysiological profiles of this patient group and correlation with symptom clusters is likely to yield useful information both about subtypes and about treatment avenues for these patients. This suggestion is also supported by the earlier conclusion by Lahmeyer et al.
2 suggesting that the use of a battery of tests could help define BPD subgroups. The application of electrophysiological test batteries is more likely to be useful than the application of a single test,
48 except when a very specific hypothesis is being tested.
Future electrophysiological investigations of BPD should strive to combine the different test modalities available and provide clinical rating scales capable of elucidating the entire array of symptoms exhibited by BPD patients. Particular attention should be paid to the problem of comorbidity, and the training and qualifications of the personnel involved in the clinical characterization of study subjects should be specified. Such personnel should be trained to a standard reliability criterion. This component of the study design will allow the examination of any correlations between biological deviations and symptom clusters. Studies should also include both healthy and patient control groups. Furthermore, based on the questions asked, researchers may consider including Axis II patient control groups. If medications are not withdrawn, patient control groups should additionally be matched for pharmacotherapy.
The need for further research is indicated by our incomplete understanding of the neurobiology of BPD as well as the clinical and research implications of the various neurobiological aberrations reported in association with this disorder, such as diminution of P300 amplitude and shortened REM latency. Physiological modalities that previously were rarely used to examine BPD (e.g., slow eye-movement tracking) have provided interesting pilot data and deserve follow-up studies. Only a few studies used quantified EEG technology to examine BPD. These studies demonstrated the potential value of this technology in probing the EEG correlates of the different symptom dimensions of BPD patients.
60,61The reported electrophysiological aberrations suggest that the application of the more recently developed investigative modalities, such as high-density EEG for precise localization of the neuronal source of different scalp-recorded potentials, magnetoencephalography for examining cortical regions not accessible by EEG, and transcranial magnetic stimulation for examination of cortical inhibitory/excitatory mechanisms, could be useful in further probing this devastating and costly disorder.
ACKNOWLEDGMENTS
This work was supported by the VA–Connecticut Healthcare System. The authors are grateful to Steven Southwick, M.D., for his assistance.