The thalamus has been referred to as the “Grand Central Station” of the brain because virtually all incoming information relays through it en route to the cortex. In turn, virtually all areas of the cortex project to divisions of the thalamus. Thus, knowledge of thalamic anatomy and connections is critical in understanding thalamic influence on cortical function and in the interpretation of functional brain imaging studies. The multiple systems for naming the divisions of the thalamus make the literature challenging.
1,2 In addition, the illustrations used in traditional texts do not easily translate into the format of clinical imaging reports. The purpose of this paper is to bypass these obstacles by summarizing and synthesizing the complex functional anatomy of the portions of the human thalamus related to memory, emotion, and arousal. Color-coding is used to facilitate identification of the relevant nuclei and their connections. Clinical images are provided to assist in translating the anatomical knowledge to the bedside.
This communication is intended as a general guide, since both the connections and the functions of these nuclei are not completely understood. While some clinically based tracing of connections has been done, most of what is known comes from experimental studies. To assure the closest possible match to human functional anatomy, only the results from human and primate studies have been used in this synthesis. In the future, imaging techniques that allow functional anatomy to be studied
in vivo in humans will provide the data needed to further refine these maps. In particular, the advent of much higher resolution magnetic resonance imaging will make fine mapping of pathway degeneration possible.
3 Diffusion tensor imaging also shows promise for both pathway tracing and perhaps identification of individual thalamic nuclei
in vivo.
1,4 It is clear that the size and boundaries of both cortical projection areas and thalamic nuclei vary across individuals.
2,5,6 Detailed studies that will eventually provide probabilistic maps of cortical functional divisions (i.e., Brodmann areas) are underway.
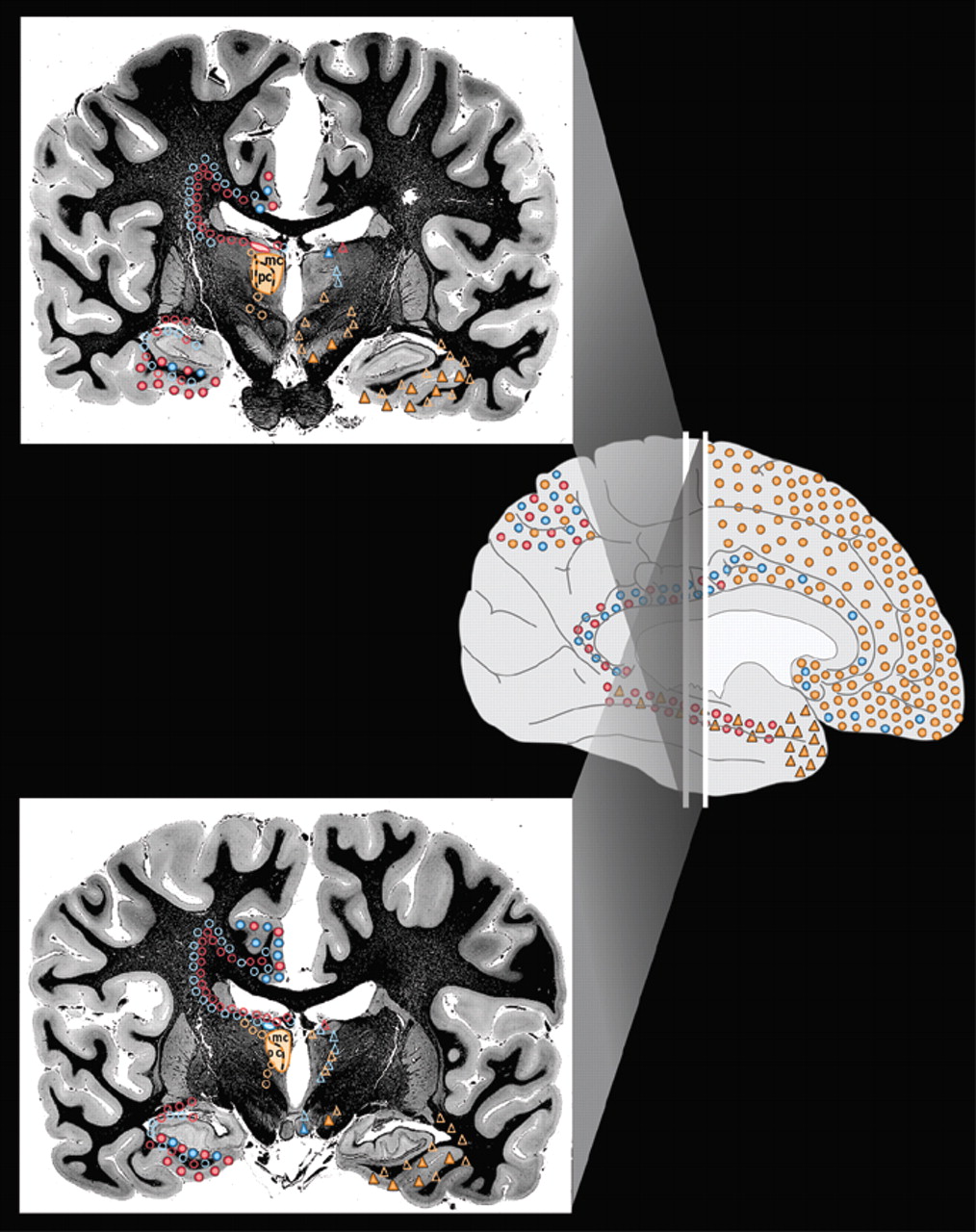
2Thalamic nuclei that are generally agreed to be involved in memory, emotion, and arousal are filled with color on the left side of the coronal brain sections in
Figure 1a and
Figure 1b. They are the medial dorsal nucleus (orange), the anterior nucleus (blue), and the lateral dorsal nucleus (pink).
7,8 These nuclei have intimate interconnections with cortical and subcortical areas (indicated by colored symbols on the coronal brain sections in
Figure 1a) that are considered part of the limbic system of the brain and deemed the “limbic thalamus.”
9 They have also been termed the “visceral thalamus” because they are vital for maintenance of circadian rhythms and for the generation of normal sleep patterns. Some authors also include the midline, intralaminar, and medial pulvinar nuclei in the “limbic thalamus” because of their projections to the cingulate cortex.
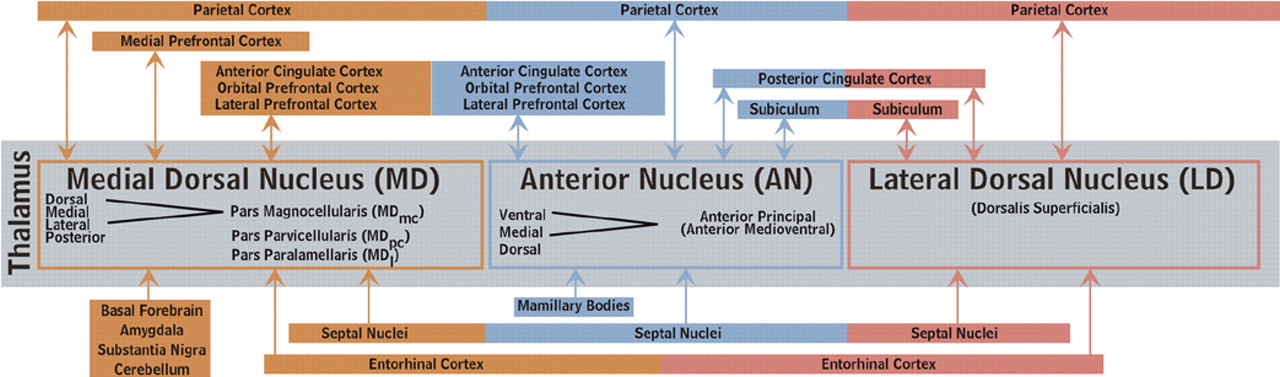
10–13The nomenclature, nuclear divisions, and subdivisions of the thalamus vary among medical specialties, a potential source of confusion when reading the literature of different fields. Two commonly used systems are summarized within the circuit diagram in
Figure 1b. Recognition of the various internal divisions of thalamic nuclei is increasingly important. The tracing of circuits within the brain has progressed to the point that function can be attributed, at least on a tentative basis, at this finer level of localization.
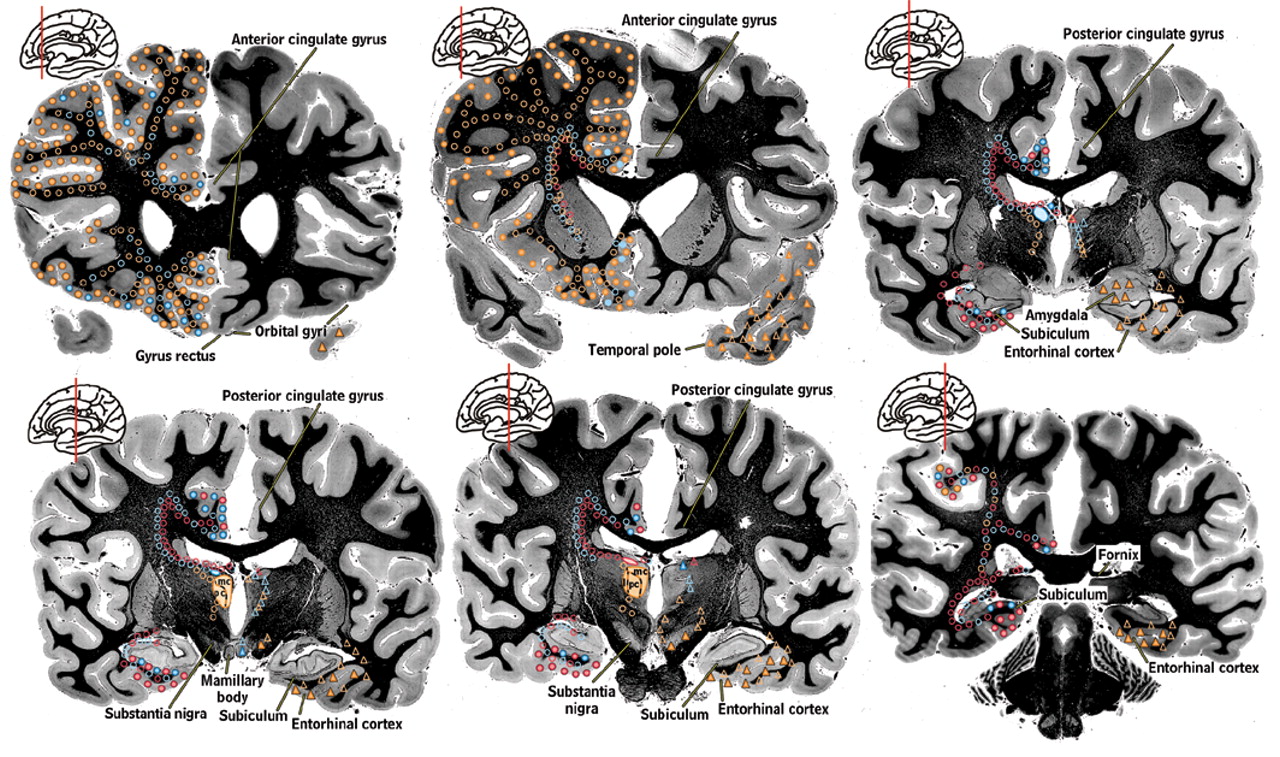
14,15 A summary of the major connections of these nuclei are color-coded onto surface drawings of the cortex (see
Cover) and images of coronal myelin-stained brain slices (
Figure 1a). Connections within both the left and right hemisphere are thought to be very similar. Connections with brainstem nuclei are not included as they are not currently thought to be specifically involved in limbic function.
Medial Dorsal Nucleus (MD)
Medial dorsal nucleus has major reciprocal connections with orbital, medial prefrontal, lateral prefrontal, and anterior cingulate cortices.
10,11,16–30 Reciprocal connections with supplementary motor and parietal cortices as well as the frontal eye fields have also been reported.
10,11,18–21,26–28 It receives input from temporal polar, entorhinal and primary olfactory cortices as well as much of the basal forebrain. This includes the septal nuclei, ventral pallidum, nucleus basalis of Meynert, and diagonal band of Broca.
10,16,20,24,25,31–38 The amygdala, substantia nigra, and cerebellum also project to MD.
19,20,24,36,38–42 These connections and the effects of injury to this region indicate a role in memory (perhaps specifically in retrieval of episodic memory), mood, motivation and the sleep/waking cycle. It has been proposed that the connections of the MD segregate into at least three major functional circuits.
14,15,43 The dorsal portion of the magnocellular part of MD (MDmc) has reciprocal connections with anterior cingulate cortex and is involved in motivation. The ventral portion of MDmc has reciprocal connections with orbitofrontal cortex and is involved in inhibition of inappropriate behavior. The parvicellular portion of MD (MDpv) has reciprocal connections with dorsolateral prefrontal cortex and is involved in mediation of executive functions.
Anterior Nucleus (AN)
Anterior nucleus has major reciprocal connections with anterior and posterior cingulate cortices and the subiculum-presubiculum portion of the hippocampal complex.
9–11,16,18,25,28,34,44–48 Lesser connections with orbital frontal, dorsolateral prefrontal, and parietal cortices have also been reported.
10,18,26–28,30,34,39,45,46,49 AN also receives major input from the mamillary complex.
8,50 It may also receive a projection from the septal nuclei.
16,36 These connections and the effects of injury to this region indicate a role in memory, modulation of the sleep/waking cycle, and directed attention.
Lateral Dorsal Nucleus (LD)
Lateral dorsal nucleus has major reciprocal connections with posterior cingulate and parietal cortices as well as the subiculum-presubiculum and entorhinal cortex portions of the hippocampal complex.
8,10,11,26,27,34,48–52 It may also receive a projection from the septal nuclei.
16,36 A role in integration of motivation and/or attention with sensory processes has been suggested.
10,53Pathology
A common source of injury to the thalamus is vascular insult. Most vascular lesions are fairly large, affecting all or part of multiple nuclei as well as associated tracts. As a result, there is a great deal of overlap in symptoms related to infarction or hemorrhage from a particular artery that can serve the anterior and medial thalamus.
54–58 Injury to the left is associated with deficits in language, verbal intellect and verbal memory. Right-sided injury is associated with deficits in visuospatial and nonverbal intellect and visual memory as well as delirium. Medial thalamus appears to be particularly important for temporal aspects of memory. Bilateral injury is associated with severe memory impairment (thalamic amnesia) as well as dementia. Some researchers attribute the memory deficits to destruction of the tracts connecting limbic structures to AN and MD (mamillothalamic tract, amygdalofugal tract). Injury to the anterior and medial thalamus can also result in disturbances of autonomic functions, mood, and the sleep/waking cycle.
59,60 Symptoms usually lessen with time, but commonly significant impairment remains.
The limbic areas of thalamus are vulnerable to nonvascular insults. Korsakoff's syndrome results from thiamine deficiency (often as a result of alcoholism). It is characterized by both anterograde and retrograde amnesia, particularly for recent events, and confusion (acutely). These symptoms have been attributed variously to involvement of the mamillary bodies, MD and AN.
3,61–63 Fatal Family Insomnia is a prion disease characterized by severe neuronal degeneration in AN and MD.
64 Pathways are not affected. This degeneration is not visible on either computed tomography or magnetic resonance imaging, but is associated with anterior thalamic hypometabolism in some cases.
65,66 There may also be widespread cortical hypometabolism. Clinically there is progressive impairment of the sleep-waking cycle progressing to total loss of sleep as well as autonomic dysfunction and gradual loss of hormonal circadian rhythms.
67,68 Prior to death the patient becomes stuporous. The clinical features may be a direct result of neuronal injury in AN and MD or may result from disconnection of the hypothalamus, limbic, and prefrontal cortices.
68Conclusion
The functional anatomy of the thalamus is both complicated and clinically important because multiple types of information pass through it on the way to the cortex. Thus, injury or dysfunction within the thalamus can alter functioning of other areas, as shown by remote alterations in cerebral blood flow and metabolism.
69–76 Conversely, injury remote from the thalamus can cause thalamic dysfunction or degeneration.
77–80 Knowledge of the functional anatomy of the thalamus should prove useful in evaluating diminished cortical functions caused by thalamic lesions. This includes, but is not limited to, patients undergoing functional imaging. It will promote understanding of thalamic lesions and hypometabolism caused by anterograde and/or retrograde degeneration following cortical injury. Additionally, it will aid in the detection of radiographically subtle, clinically significant lesions; in the planning of rehabilitation and psychiatric treatment; and in the planning of magnetic resonance imaging-guided thalamic surgery.
ACKNOWLEDGMENTS
This study was supported by a grant from the Mike Hogg Fund to Dr. Taber. The authors thank Archibald J. Fobbs, Jr., B.S., curator of the National Museum of Health and Medicine, Armed Forces Institute of Pathology, Washington, D.C., for providing the myelin-stained coronal brain slices.