Depression is a common, treatable, yet often unrecognized complication of stroke, and it is associated with poor functional
1–3 and cognitive
4 outcome and, possibly, increased mortality.
5 The recognized risk factors for poststroke depression (PSD) include previous depressive episodes
6 and stroke severity and disability.
3 However, the significance of stroke lesion location for the consequent depression remains an unsolved question and has been disputed for more than a decade. The prevalent hypothesis of increased risk for PSD involving lesions that affect anterior brain areas with left side predominance
7–10 has been recently challenged by exhaustive reviews in this area.
11,12 In their meta-analysis, Carson et al.
11 found no association between brain infarct localization and depression. However, many of the previous studies addressing the significance of lesion location in PSD have methodological problems (i.e., those resulting from small patient samples and unrepresentative patient populations). The radiological data are based almost exclusively on computer tomography technology, which is less sensitive than the magnetic resonance imaging (MRI) method in detecting vascular changes and their locations within gray and white matter structures.
We have assessed the relations between PSD and vascular changes detected with MRI in the Helsinki Stroke Aging Memory Study,
13 which addresses a subpopulation of our previous patient group. In our previous study, we did not exclude patients who had no detectable infarcts or who had more than one infarct as detected by MRI. We found that patients with depression (
n=109) had a higher proportion and larger volume of infarcts that affected the prefrontal-subcortical circuits (especially the caudate, the pallidum, and the genu of the internal capsule, with a left-side predominance) than did patients without depression (
n=166). The independent correlates of poststroke depression were the mean frequency of infarcts in the genu of the internal capsule on the left side (odds ratio [OR]=3.2), the mean frequency of infarcts in the pallidum on any side (OR=1.6), and the mean volume of infarcts in the right occipital lobe (OR=0.98). The extent of white matter lesions (WMLs) and atrophy did not substantially differ for those patients with and without depression. The patients had 3.2 brain infarcts on average. In this field, however, the standard procedure has been to include only patients with exactly one infarct visible by computed tomography (CT). We chose to use the same approach in the present study in order to compare our results with those obtained earlier by the CT method. The relationship between lesion location and poststroke depression was expected to be more prominent in a patient population with only one stroke when studied with the more sensitive MRI method.
METHODS
The Helsinki Stroke Aging Memory Study was conducted in Helsinki University Central Hospital in Helsinki, Finland between December 1993 and March 1995. The procedures of the study have been detailed.
3,4In the psychiatric subarm of the study, 275 consecutive patients aged 55 to 85 years underwent both extensive psychiatric evaluation and comprehensive MRI ratings at 3 to 4 months after ischemic stroke. Only one brain infarct, clinically related to each patient's stroke symptoms, was detected in each MRI scan of 70 subjects (25.4%), who form the present study population. The protocol included a detailed structured clinical interview of the patient and a knowledgeable informant and a structured clinical and neurological examination by board-certified neurologists (T.P. and R.V.). The cases were also reviewed by a senior neurologist (T.E.). Cognitive function was assessed by the Mini-Mental State Examination (MMSE),
14 stroke severity by the Scandinavian Stroke Scale,
15 aphasia by the Acute Aphasia Screening Protocol,
16 and impairment in activities in daily living (ADLs) by the Barthel Index.
17 Patients requiring daily assistance, home attendant help, or admission to a nursing home were classified as dependent, as defined by Tatemichi et al.
18Clinical psychiatric examination (by A.L.) included a computer-assisted structured interview, including the Present State Examination
19 and Schedules for Clinical Assessment in Neuropsychiatry.
20 For the final DSM-III-R
21 and ICD-10
22 diagnoses of depressive disorders, all psychiatric data were combined. Depressed patients without any recent psychosocial stress factors except stroke were defined as having stroke-related depression. We included patients with minor and major depression. The patients with depression poststroke included subjects with DSM-III-R major depressive disorder (single or recurrent episode), bipolar disorders (depressed episode), organic mood disorder (organic depressive disorder), adjustment disorder (depressed or mixed anxiety and depressed mood), dysthymic disorder, dementia with depressed mood (as defined in DSM-IV
23), and major depressive disorder in partial remission.
Patients with no visible brain infarct (n=13) and patients with more than one brain infarct (n=192) on MRI were excluded. Excluded patients did not differ from included patients (n=70) with regard to sex, age, or functional status in ADLs, as assessed by the Barthel Index (data not shown). Neither the cognitive differences, as defined by MMSE score (excluded versus included, mean=25.7, SD=4.0, versus mean=26.0, SD=4.0) (P=0.45, Mann-Whitney test), nor percentage of patients with DSM-III-R diagnosis of dementia (excluded versus included, 19.6% versus 15.4%) (P=0.58, chi-square test) reached statistical significance between the two groups.
The radiological method has been described in more detail in a previous study.
24 In short, MRI obtained for research purposes was performed with a 1.0 T system. All images were reviewed by the same neuroradiologist (R.M.), who was blinded to the clinical data and not involved in the clinical care of the patients. The number, type, side, site, and size of focal lesions were recorded. Lesions equivalent to the signal characteristics of cerebrospinal fluid on T1-weighted images and measuring over 3 mm in diameter, as well as wedge-shaped cortical-subcortical lesions, were regarded as brain infarcts. The anatomical sites included: 1) brain lobes (cortical-subcortical lesions in frontal, temporal, parietal, and occipital lobes; 2) vascular territories (deep and superficial anterior cerebral artery, middle cerebral artery, posterior cerebral artery, internal cerebral artery and border-zone areas); and 3) specific locations (medulla, pons, cerebellum, optic radiation, thalamus, caudate, putamen, pallidum, genu of internal capsule, anterior and posterior capsule, anterior and posterior corona radiata, anterior and posterior centrum semiovale, genu, body and splenium of corpus callosum, angular gyrus, hypothalamus, hippocampus and amygdala). The prefrontal-subcortical circuit
25 includes connections between the frontal cortex, caudate, pallidum, thalamus, genu of internal capsule, anterior capsule, anterior corona radiata, and anterior centrum semiovale. We compared the number of patients with infarcts affecting these sites in patients with and without depression. The location of the infarcts was recorded at either side (i.e., the pallidum of either side), and then the side was recorded in order to determine the significance of laterality (
Table 2). We also compared the estimated size of the lesions between these two patient groups. The lesions were grouped into four categories based on their largest diameter (3 mm to 9 mm, 10 mm to 29 mm, 30 mm to 59 mm, and over 60 mm), and the radii used for calculations were 3 mm, 10 mm, 20 mm, and 30 mm, respectively. The volume of the lesion was then estimated by a formula for calculating the volume of the ball.
White matter lesions were rated on proton density-weighted images in six areas: around the frontal and posterior horns and along the bodies of lateral ventricles; and in subcortical, deep, and watershed areas. Periventricular WMLs around the frontal and posterior horns were classified according to size and shape into small cap (≤5 mm), large cap (6 to 10 mm), and extending cap (>10 mm), and WMLs along the bodies of lateral ventricles were sorted according to thin lining (≤5 mm), smooth halo (6 to 10 mm), and irregular halo (>10 mm). White matter lesions in the subcortical, deep, and watershed areas were classified based on size (greatest diameter) and shape into small focal (≤5 mm), large focal (6 to 10 mm), focal confluent (11 to 25 mm), diffusely confluent (>25 mm), and extensive WML (diffuse hyperintensity without distinct focal lesions affecting the majority of white matter area). The number of each type of hyperintensity was counted, and extensive WMLs were rated as present or absent. Moderate and severe degrees of WMLs included large and extending caps at a periventricular area; smooth halo and irregular halo along the bodies of lateral ventricles; and focal confluent, diffusely confluent, and extensive WMLs in the subcortical, deep, and watershed areas. The extent of WMLs was graded with the 4-point scale of Fazekas et al.
26Brain atrophy was first rated as none, mild, moderate, or severe by comparison to standard images. Cortical atrophy was rated in frontal, parietal, and occipital lobes; central atrophy in temporal, frontal, and occipital horns, and bodies of lateral ventricles; and medial temporal lobe atrophy in entorhinal cortex and hippocampus. Cortical and central atrophy was expressed as the mean of the rating in all the bilateral areas rated and divided into two groups: none to mild versus moderate to severe. The intraobserver reliabilities for rating WMLs and atrophy were tested and found to be suitable (WML, weighted kappa=0.90 to 0.95; atrophy, weighted kappa=0.75 to 0.82).
In the statistical analysis we compared patients with and without PSD. The chi-square test and Fisher’s exact test (two-tailed) were applied for categorical data, and the t test and Mann-Whitney were used for continuous data. The radiological variables that significantly differentiated the patients with and without PSD are presented in
Table 2 and
Table 3. These variables were chosen for a multiple logistic regression analysis in order to find the independent MRI correlates of PSD. The statistics were analyzed with the BMDP
27 and SAS
28 programs.
The study was approved by the Ethics Committee of the Department of Neurology, Helsinki University Central Hospital, Helsinki, Finland. The study design was thoroughly explained to all participants, and written, informed consent was given.
RESULTS
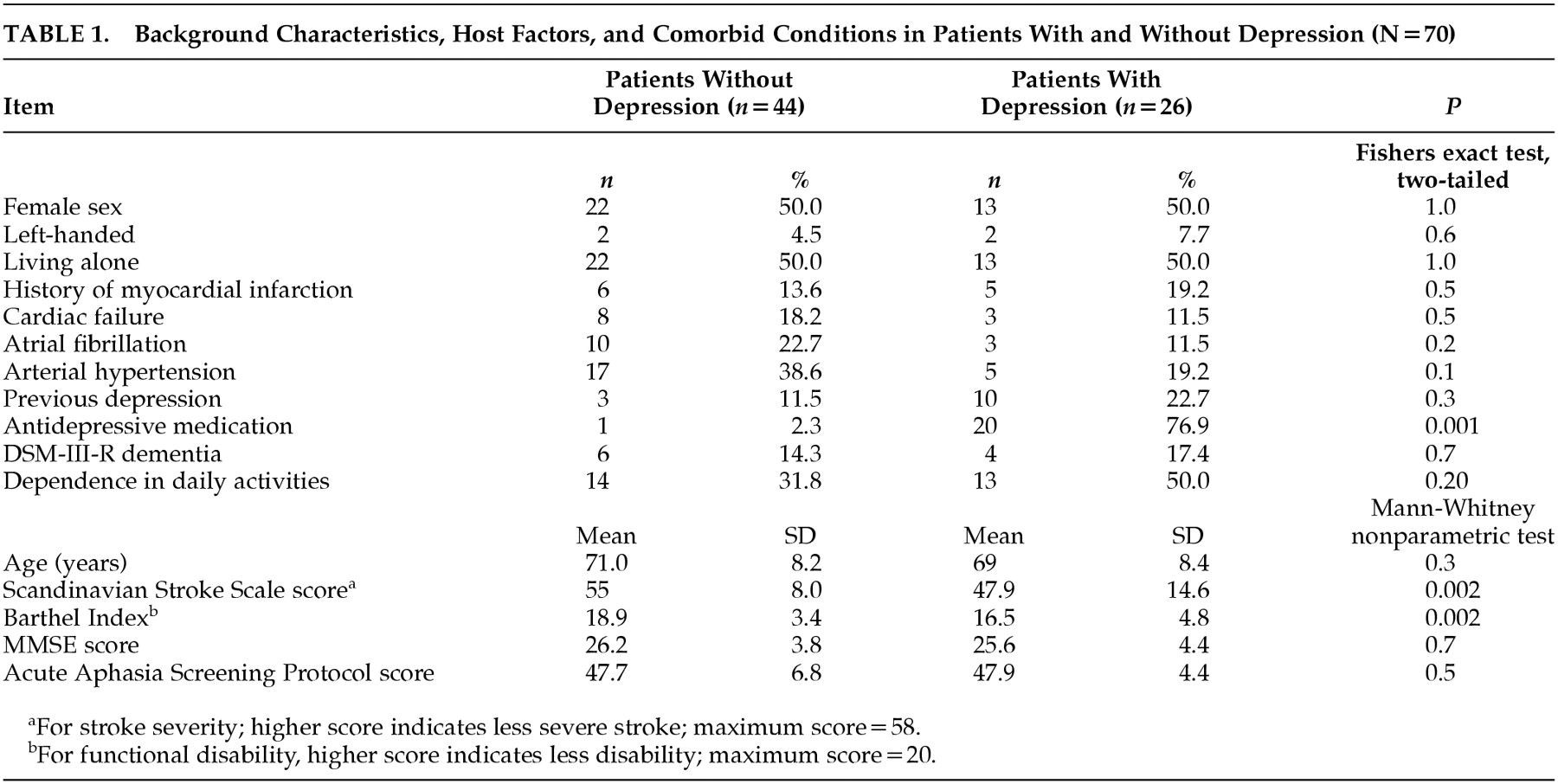
Stroke-related depression was present in 26 (37.1%) of the 70 patients. Background characteristics, host factors, and some comorbid conditions are presented in
Table 1. The depressed patients were more impaired in activities of daily living, as indicated by the difference in the Barthel Index Score; had more severe stroke, as indicated by the Scandinavian Stroke Scale score; and were taking antidepressive medication more often.
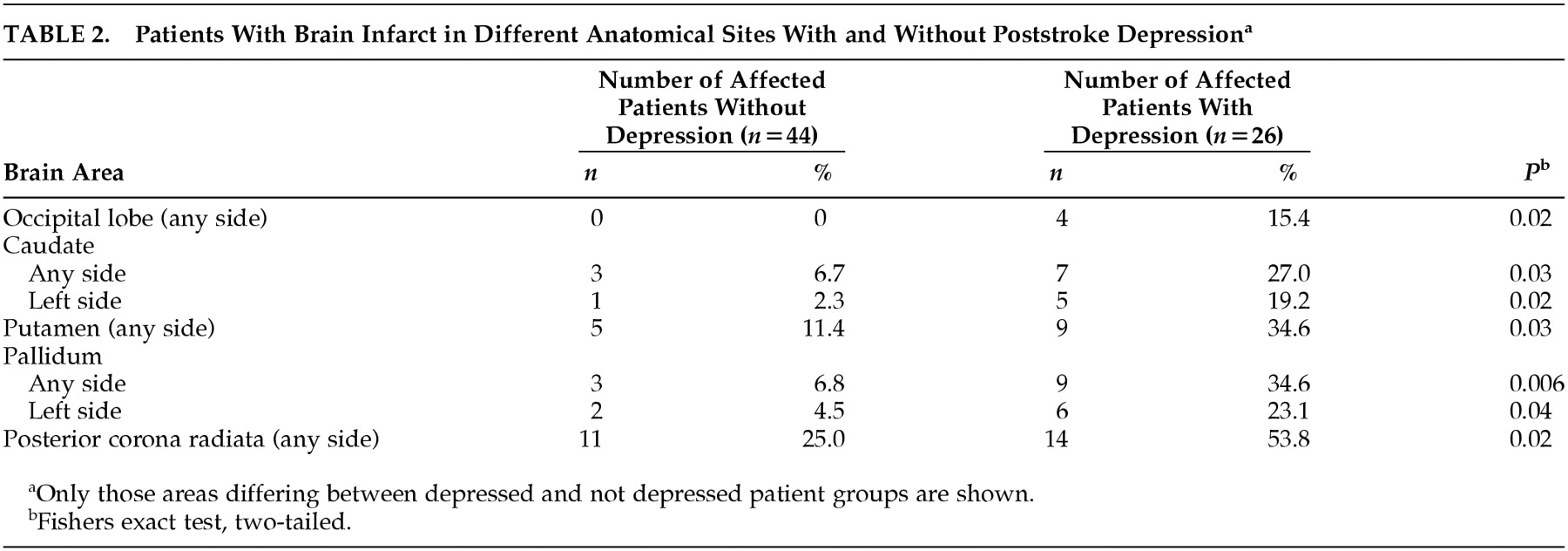
In patients with PSD, the brain infarct significantly affected basal ganglia more often, mainly caudate, putamen, and pallidum, as well as posterior corona radiate. The sites that differed significantly between the depressed and nondepressed patient groups are shown in
Table 2.
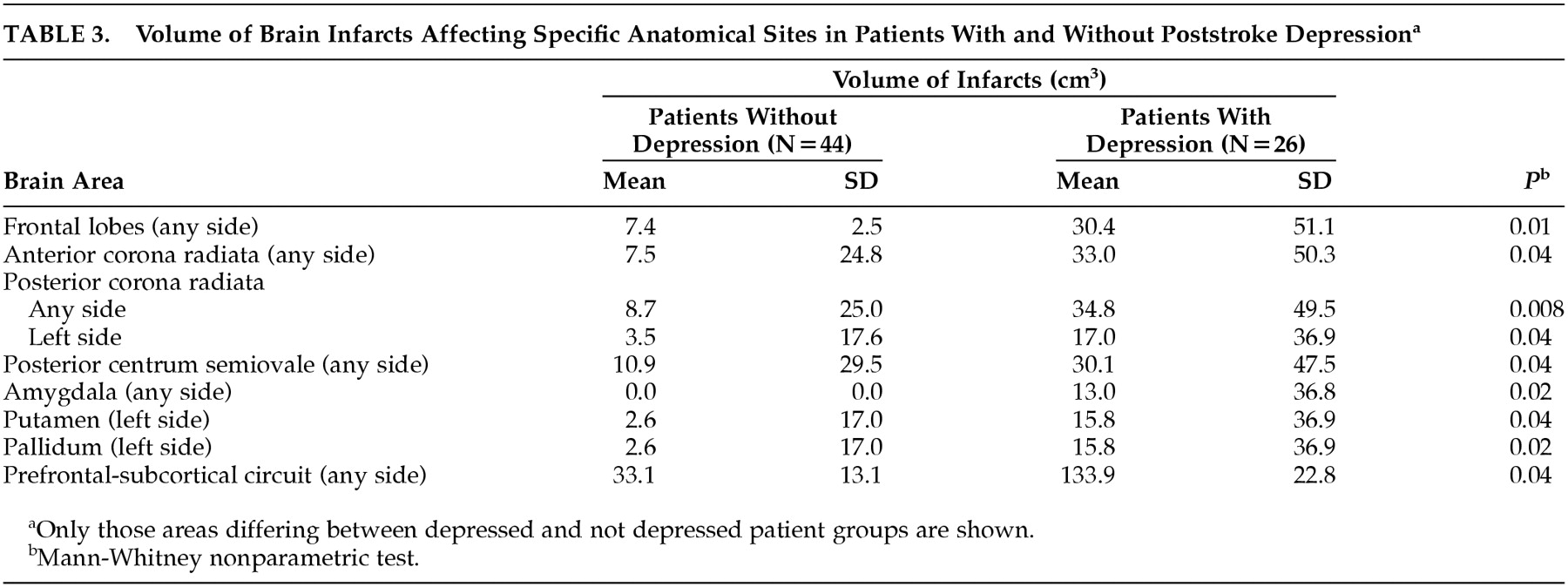
The total volume of the brain infarct tended to be larger in patients with poststroke depression, but the difference between the nondepressed and depressed patient groups was just outside the limit of significance (mean=17.9 cm
3, SD=29.7, versus mean=36.7 cm
3, SD=48.7) (p= 0.05, Mann-Whitney test). The sites with larger brain infarcts in depressed patients are shown in
Table 3.
No difference existed in the extent of WMLs in the periventricular areas, centrum semiovale, subcortical areas, watershed areas, or the mean Fazekas WML score between the depressed and nondepressed patient groups. Further, no difference appeared between the two groups in the grade of cortical, central, or medial temporal brain atrophy (data not shown).
In the logistic regression model, the only independent correlate of PSD was the infarct localization in the pallidum of either side (OR=7.2; 95% confidence interval=1.9–35.7, SE=0.73; coefficient=1.9).
DISCUSSION
Evidence supporting the significance of the frontal lobes and the basal ganglia in depression is accumulating from the structural MRI and CT studies,
29 functional PET and MRS studies,
30–32 and clinical studies of disorders that affect these structures (e.g., Parkinson's disease,
33 Huntington's disease).
34 The probable neurobiological mechanism of the affective changes in these disorders involves the dysfunction of the prefrontal-subcortical neuronal loops that control emotion and behavior.
25 Accordingly, it is reasonable to assume that depression is a result of a brain infarct or infarcts that disrupt these loops. However, the review by Carson et al.
11 stated that evidence for this theory is not convincing. The principal results of the present study once again suggest that infarcts within critical anatomical substrates of the prefrontal-subcortical circuits, namely the caudate and pallidum, are associated with PSD. When both hemispheres were analyzed separately, the lesions affecting these structures on the left side were associated with PSD, whereas right-sided lesions were not. Other critical areas were the putamen, posterior corona radiata, and left occipital lobe. The size of the brain infarct also correlated with poststroke depression in our patients. Lesions affecting the striatum in the left hemisphere or the structures containing projections there—the centrum semiovale or corona radiata on either side—were larger in the depressed patients. Moreover, the brain infarcts affecting the frontal lobes, amygdala, putamen, or any part of the prefrontal-subcortical loop were larger in patients with PSD. In the multivariate model, only the localization of the infarct in the pallidum was an independent correlate of PSD. The statistical association of pallidum infarction and poststroke depression was stronger in this study than in our previous study,
13 in which we included patients with any total number of MRI-detected brain infarcts. This is in accordance with our study hypothesis. Taken together, our results support the idea that there are critical lesion locations—the pallidum being the most prominent—for the development of PSD, and left-sided lesions are more often involved.
The concept of vascular depression,
35 supported by data from geriatric depression patients,
36 suggests that ischemic white matter changes affecting the frontal-subcortical circuits may correlate with depression. Our approach is different, as we studied patients with established stroke: an end-stage of ischemic vascular disease. Thus, our finding of absence of correlation between poststroke depression and severity or localization of WMLs does not mean that white matter changes may not contribute to depression in elderly people in general. Likewise, although we found no correlation between poststroke depression and degree of brain atrophy, one must be mindful that the single-infarct patients in this study represent only a small fraction of the patients with ischemic vascular disease.
It has been proposed that major and minor depression after stroke are two distinct disorders.
37,38 This subject is still controversial, however, as some of the somatic symptoms caused by stroke per se can be misidentified in DSM classifications as part of a depression syndrome.
39 We also find diagnosing major versus minor depression in poststroke elderly patients using DSM criteria difficult. These patients often have dementia, communicative disorders, dysexecutive symptoms, or apathy that may mimic or obscure depressive symptoms. Before working diagnostic criteria can be developed, the concept of PSD needs to be reframed so that these features are better understood. At this stage, we did not want to distinguish different depressive syndromes, but included patients with any depressive disorder.
What is the reason for the discrepancy between our results and the conclusion of the meta-analysis of Carson et al.?
11 First, in most of the studies included in their review, the localization of the brain infarcts has been coarse or inaccurate. The most prominent method for localization has been one developed by Robinson and Szetela
40 in which the lesion location in the anterior-posterior axis was calculated as the distance from the anterior pole in millimeters. Although this method probably has neurobiological validity by reflecting the anterior orientation of the architecture of emotion control, its neuroanatomic basis is vague. By use of such procedures, the significance of the damage of minute subcortical structures will be missed. Likewise, if neuroanatomical substructures are not taken into consideration, the significance of lesion laterality remains concealed. The present study showed no significant differences between lesion location in either of the hemispheres, but when the specific sites were analyzed separately, differences in laterality emerged. In a paper by Singh et al.,
41 the lesion location was determined on CT scans by use of a stereotactic atlas in addition to the traditional measurement of the lesion proximity to the anterior frontal pole. They found that inferior frontal lesion location, irrespective of side, was a risk factor for depression, but concluded that depression probably resulted more from functional dependence. Although the atlas-based methodology is clearly an improvement in the localization of the lesions, the smaller spatial resolution and less sensitive lesion detection of CT than of MRI may explain the differences between their findings and ours.
Another explanation for the discrepancy between our results and those summarized in the Carson et al. meta-analysis is the quality of psychiatric methodology. In diagnosing depression in stroke patients, reliable psychiatric assessment is essential. In these elderly individuals, cognitive disturbances or incipient vascular dementia may cause difficulties in concentration, apathy, loss of interest, or psychomotor retardation, in addition to the somatic symptoms of weight loss and sleep disturbances. A careful structured psychiatric methodology is clearly warranted for these patients rather than a vaguely defined clinical examination using DSM criteria or—even less recommendable—depression scales in diagnosing depression.
Carrying out a single-lesion study using MRI protocol is a challenging task. In a clinical curriculum, patients with only one brain infarct on MRI are a minority as subclinical (i.e., “silent”) brain infarcts are often detected, even in those patients with their first-ever clinical stroke. Of our series of 275 consecutive stroke patients who underwent both psychiatric examination and MRI, only 70 (25.4%) had one brain infarct. In future single-lesion MRI studies, large series of stroke patients will be needed for meaningful statistical analysis to confirm results such as ours, indicating that lesions of specific neuroanatomic structures contribute to PSD.