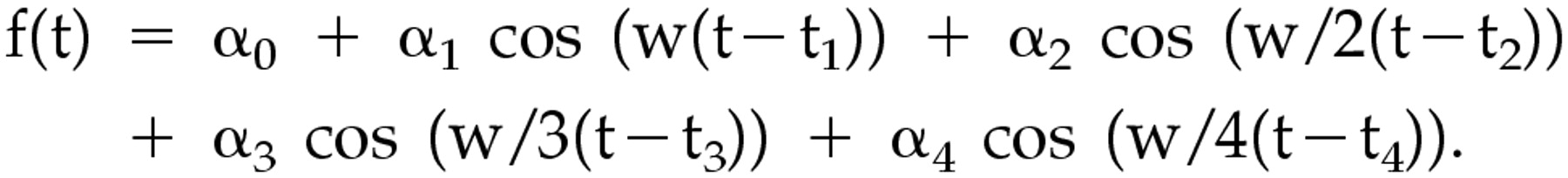Dysfunction of the circadian timing system has long been suspected in Alzheimer's disease (AD) because such patients frequently have sleep disturbance.
1–3 A limited number of studies
2,4–6 suggest that agitated behaviors in demented elderly patients, with the peak of agitation occurring just after sunset, which has been called “sundowning,” are associated with the underlying circadian rhythm. Despite such sleep disruption, some studies of various physiological phenomena, typically showing diurnal variation, have difficulty revealing differences between AD patients and controls.
7,8 Sleep disturbance, which could affect the maintenance of mood, alertness, and cognitive performance during the day, causes further clinical impairment in dementia patients and greater caregivers' burden.
9 This problem usually results in the institutionalization of dementia patients, contributing to the continued disruption of circadian rhythms.
10The suprachiasmatic nucleus in anterior hypothalamus, which was indicated as the circadian clock in previous studies, has shown significant degeneration of the vasopressin-containing neuron in aging and AD.
7,11 This may be related to the change of circadian rhythm in aging, possibly explaining the sleep disturbance in the elderly.
12–15It has been shown that age-related changes in circadian sleep wake-organization are accentuated in AD.
16,17 However, studies of body temperature rhythm have not supported a parallel decrease in rhythm amplitude.
2,18 The changes of circadian rhythm in aging did not show consistent results in the previous studies. The sleep-wake rhythm becomes polyphasic with aging due to fragmentation of nocturnal sleep and increased napping during the day. Additionally, the amplitude reduced along with decreased activity in the elderly.
19–21 Amplitude of temperature rhythm decreased with aging in the entrained condition, but not in the free-running condition.
12,13In our study, we aimed to assess whether there are any differences in circadian rhythms of sleep-wake cycle and temperature between AD patients and normal aged controls in the entrained condition. There are a limited number of studies regarding dementia patients residing at their homes (in their natural environments) rather than in nursing homes. We also analyzed the phase relationship between the two rhythms in the entrained condition in order to find any change in the circadian organization in elderly patients with AD.
METHODS
Seven patients with AD (age: 77.0±4.3, three men and four women) and 11 normal comparison subjects (age: 74.2±5.2, four men and seven women) who were registered with the Alzheimer Center at Fairhill Institute for the Elderly, affiliated with the University Hospitals of Cleveland, participated in this study. The mean age in the AD group was not significantly different from that of the comparison group. The diagnosis of AD was made according to the NINCDS-ADRDA criteria using the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Assessment Packet.
22 On the Clinical Dementia Rating (CDR) Scale,
23 AD patients received a score of 1 or 2. Subjects who had known psychiatric disorders or were taking any psychotropic drugs during the study were excluded. All subjects were free of investigation drug use at least 1 month prior to entrance into the study. All comparison subjects were evaluated by the CERAD Assessment Packet and found not to have cognitive decline, psychiatric disorders, or physical disorders.
Prior to measuring the sleep-wake and temperature rhythms, a sleep-log diary was given to each subject. Continuous measuring of wrist activity and skin temperature on the axillary region was conducted by a Mini-Logger™ ambulatory monitoring device (Mini-Mitter Company, Oregon, USA) for 96 hours (4 consecutive days and nights), starting at 8 a.m. on the first day of monitoring for each subject. Previous validation studies indicated that agreement rates between actigraph-based and polysomnograph (PSG)-based minute-by-minute sleep-wake scoring were very promising for normal subjects (above 90%).
24 The ability to document rest-activity circadian rhythmicity from actigraphic data has been well demonstrated in several studies.
25–27 Subjects were allowed to perform routine activities as usual during the monitoring period and asked to remove the actiwatch and skin temperature probe only while showering. An activity log was kept during the ambulatory monitoring in order to record sleep-wake cycles and the times in which the monitoring device was removed. Informed consent was obtained after the procedure had been fully explained.
Every minute of wrist activity was scored as either wake or sleep, as described by Cole et al.
28 Every 16-minute interval was labeled as wake if 8 or more minutes were scored as wake and as sleep if 9 or more minutes were scored as sleep. The mean temperature was calculated for the 16-minute interval.
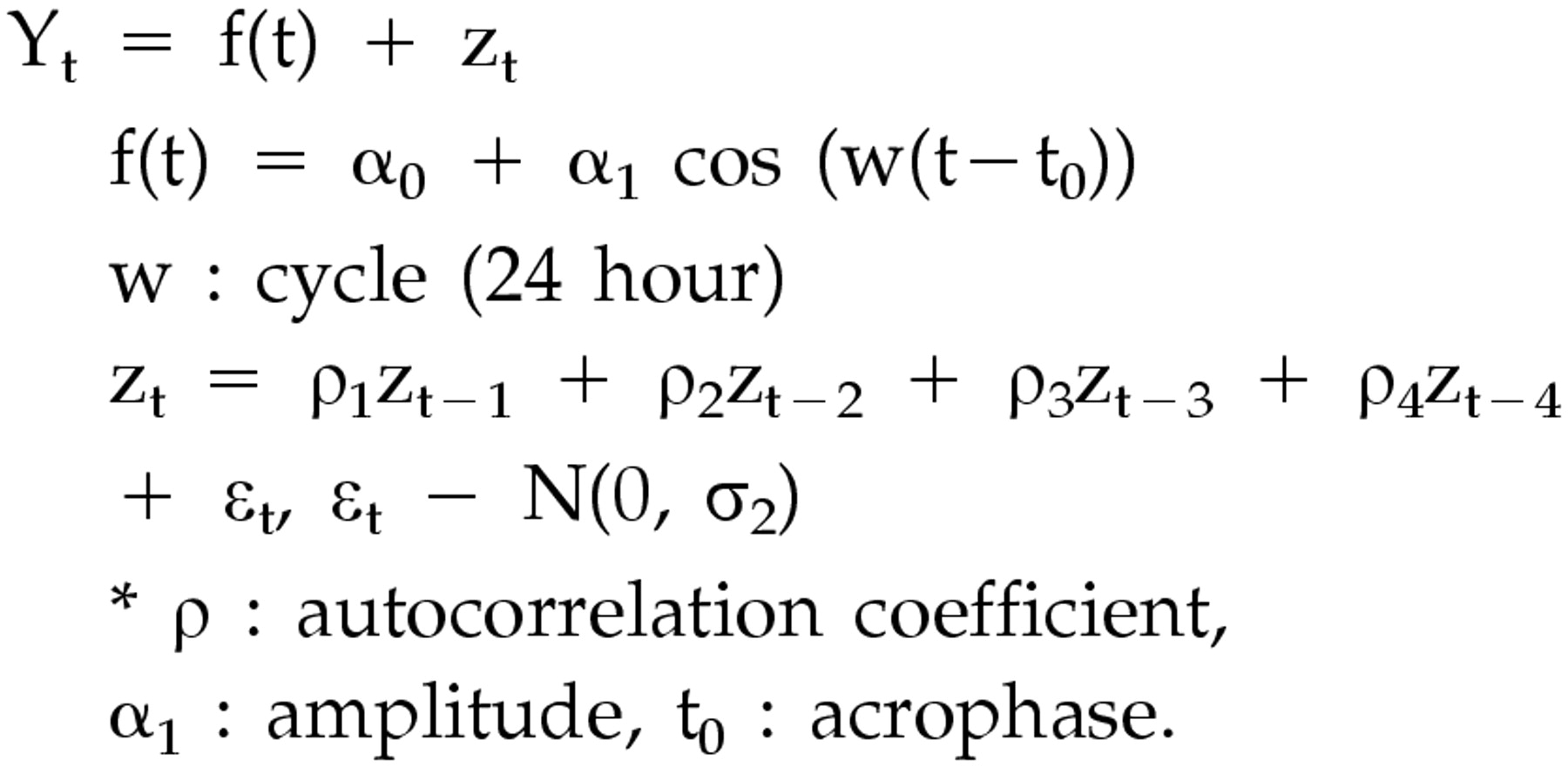
For the statistical analysis of the sleep-wake and temperature rhythms of each individual, a cosinor analysis was applied to calculate the circadian rhythm measures: acrophase (phase angle of the maximum value of a sine function fitted to the raw data of a rhythm) and amplitude (difference between maximum and mean value in a sinusoidal oscillation.
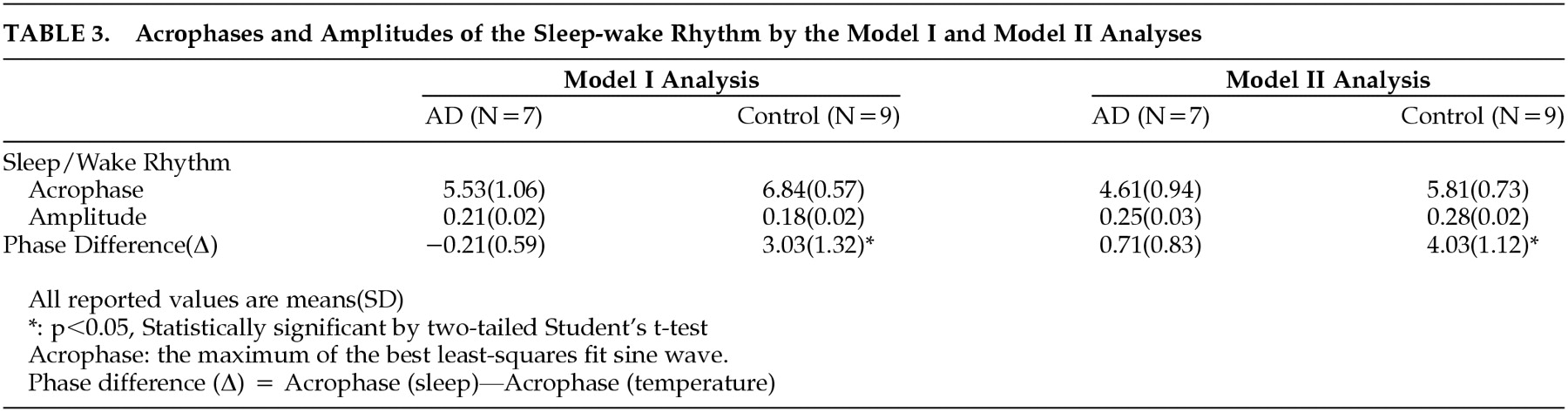
29 Since the raw data of wrist activity were presented as 0 or 1 (wake or sleep), a noncontinuous variable, the moving average technique was added. We called it Model 1, which has the following functional formula for curve fitting of the 24-hour rhythm.
Since we found that the cosine curve (dotted line) by the Model 1 analysis was not well suited to our actual data of the 24-hour sleep-wake rhythms (
Figure 1), we applied the Model 2 analysis, which has the formula below. The actual line by Model 2 analysis was different from the dotted one by Model 1 analysis.
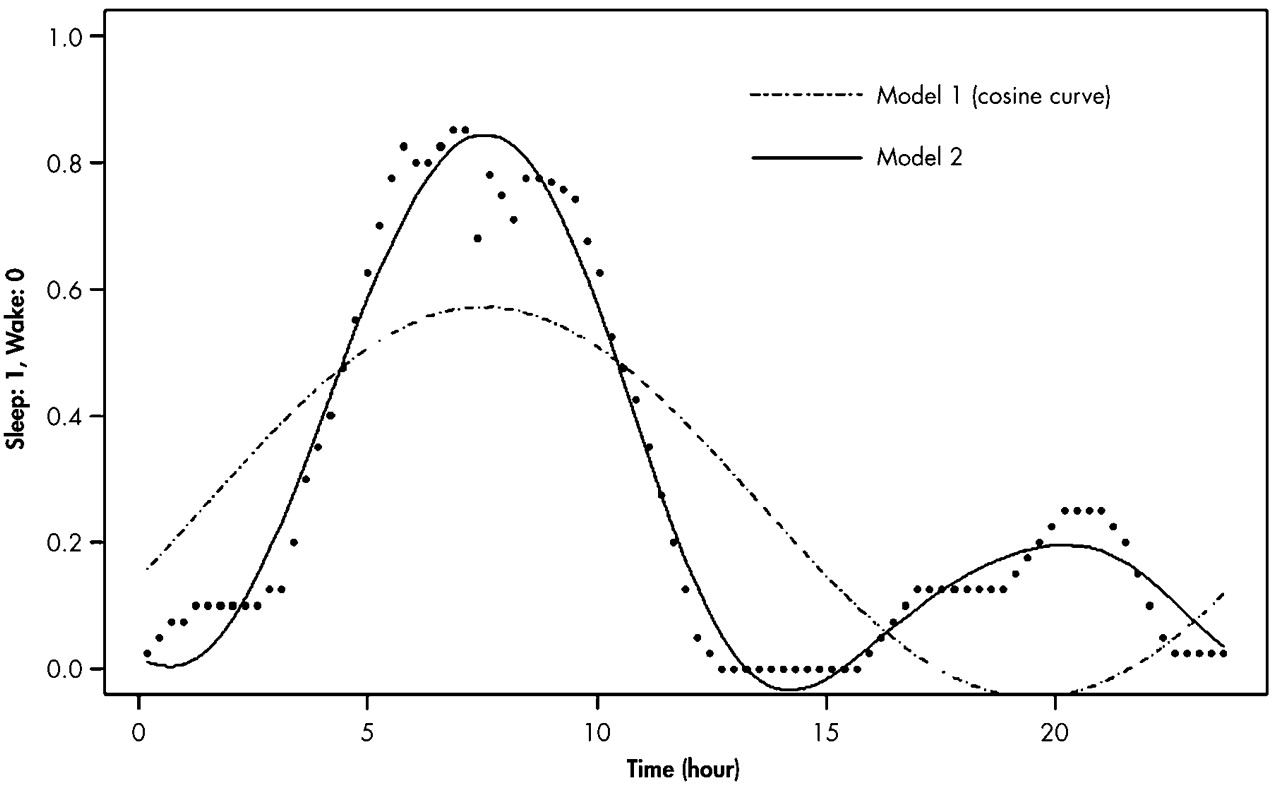
We also used the Model 1 analysis for the temperature rhythm (
Figure 2). For each individual, curve fittings of both wrist activity and skin temperature measures were conducted from the data averaged with respect to the time of day. To compare the mean acrophase and amplitude of the AD group with those of the normal group, Student's t test was performed. We determined the phase difference between the sleep-wake rhythm and temperature rhythm in each subject and compared the means of the AD and comparison groups. Two comparison subjects, whose data of wrist activity were mostly 0, were excluded from the analysis for the sleep-wake rhythm because curve fittings could not be done for them.
RESULTS
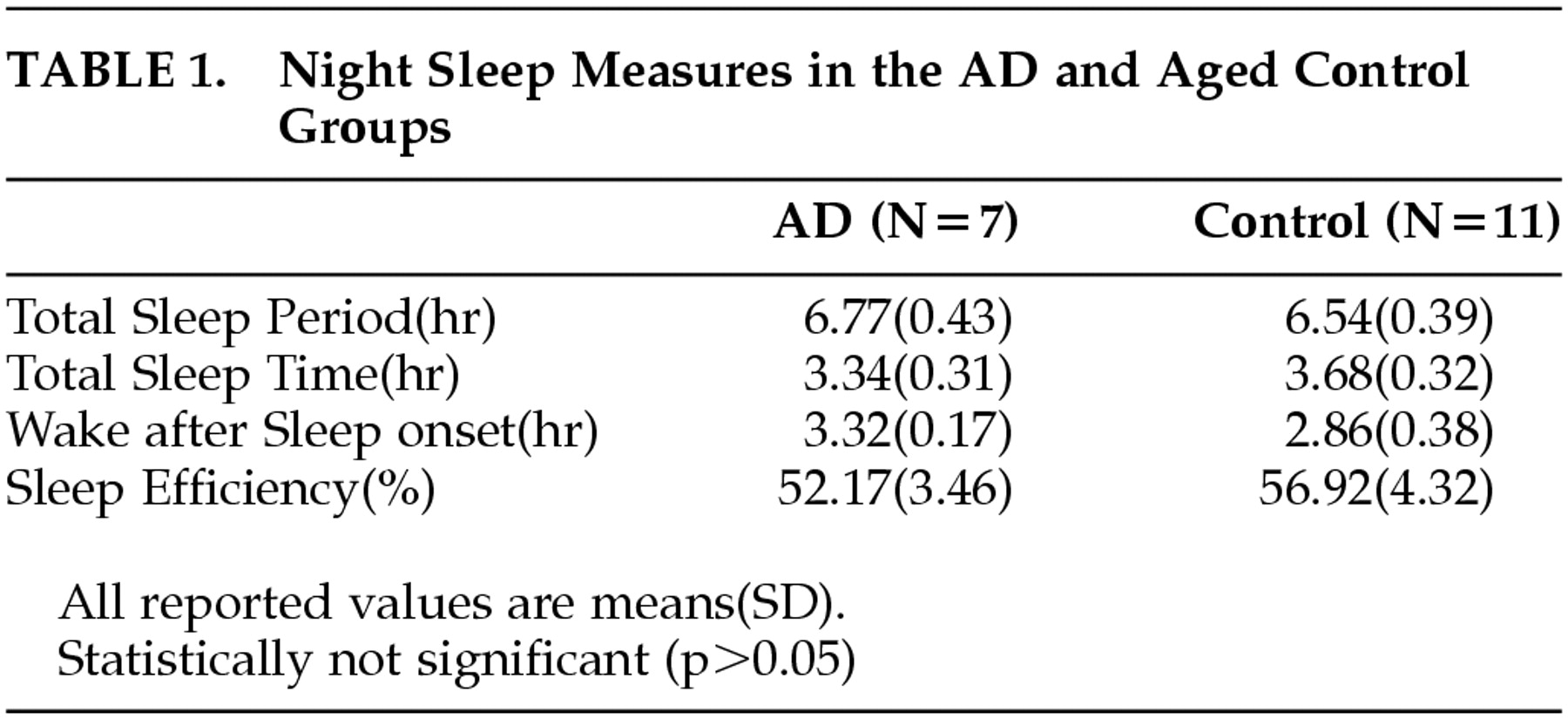
Table 1 shows the comparison of the night sleep measures analyzed from the rest-activity data between the AD group and the comparison group. The sleep measure of each individual was calculated for each day, and the average of these values for 4 days in each individual was used for obtaining the means of both groups. There was no statistically significant difference in the sleep measures (e.g., total sleep period, total sleep time, wake-after-sleep onset, sleep efficiency) between the two groups (
p > 0.05), although the mean of sleep efficiency was slightly lower, and the mean of wake after sleep onset slightly greater, in AD patients relative to those in comparison subjects.
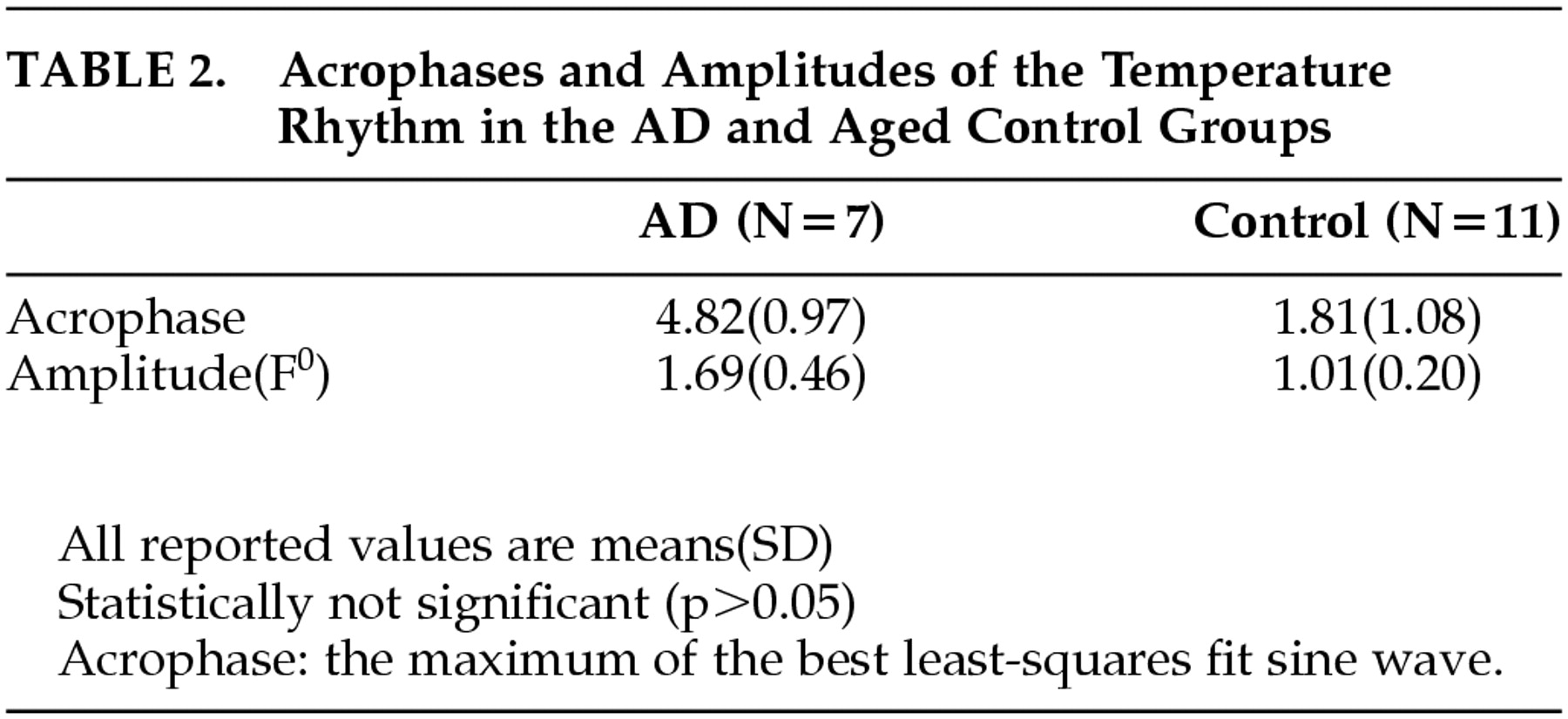
The difference in the mean acrophases of the temperature rhythm between the AD group and the comparison group was almost 3 hours (
Table 2). However, it was not statistically significant (
p = 0.05). Additionally, there was no difference in the mean amplitude between the two groups.
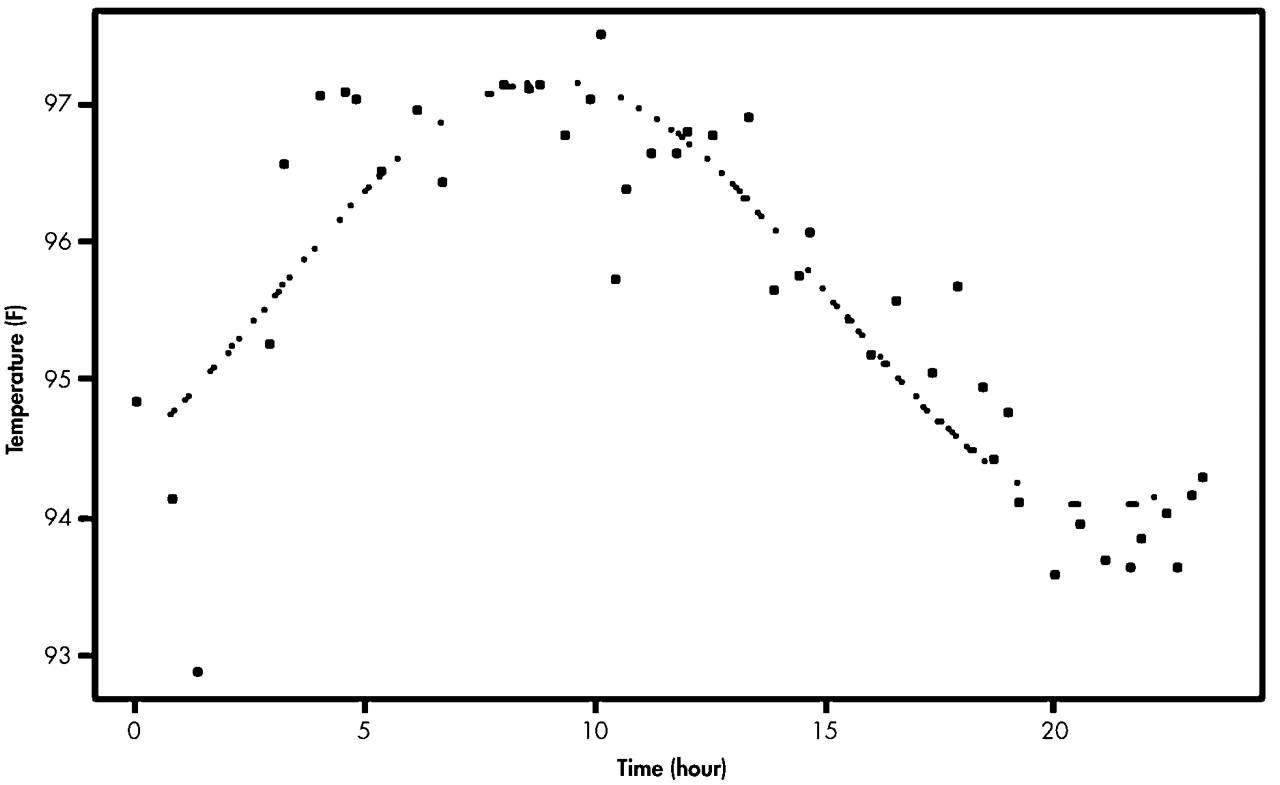
Table 3 shows the mean acrophases and amplitudes of the 24-hour rhythm of the sleep-wake cycle in both groups, which were the results taken from the Model 1 and Model 2 analyses. There was about 1-hour difference between the mean acrophases of the AD group and the comparison group. This result was similar to that taken from the Model 2 analysis. However, no significant difference in the mean acrophases between the two groups was found (
p > 0.05). There was also no significant difference in the mean amplitude of the sleep-wake rhythm between the AD and comparison groups, according to both Model 1 and Model 2 analyses, although the absolute values of the two groups in Model 1 were different from those in Model 2.
The phase difference, the difference in the acrophases between the sleep-wake rhythm and temperature rhythm was significantly lower in the AD group than in the comparison group. This shortening of the mean phase difference in the AD group appears to be attributed to the phase delay in the temperature rhythm (
Table 2) and the phase advance in the sleep-wake rhythm (
Table 3), when compared to the rhythms in the normal group. Additionally, the phase relationship between the two rhythms in the AD group by the Model I analysis showed a reverse relationship, in which the acrophase of the sleep-wake rhythm was earlier than in the temperature rhythm. The phase relationship by the Model 2 analysis, however, showed only the shortening of the phase difference between the two rhythms, keeping the same trend in the phase relationship.
DISCUSSION
Our results showed that there were no differences in the acrophases and amplitudes of the 24-hour sleep-wake and temperature rhythms between the AD and elderly comparison groups. Previous studies regarding the circadian rhythm in aging and dementia have suggested that the real manifestation by the circadian oscillators would be masked in the entrained condition. The decrements of the circadian period and amplitude of the body temperature rhythm with aging, which had been found in the entrained condition, were not observed in the free-running stage. However, in the experimental condition of constant routine, which unmasks the endogenous circadian oscillator, the circadian period and the phase relationship with the zeitgeber in the temperature rhythm was shortened in aging.
12,30 This finding could be associated with the phase advance of body temperature rhythm in the elderly, resulting in the internal desynchronization. The amplitude reduction of the circadian temperature regarding the sleep consolidation was also noted in individuals in the healthy aged group who experienced a 6-hour phase advance in constant routine.
13Our study was conducted in the usual entrained condition for the subjects who were residing at home and continuing their routine daily activities, while monitoring the sleep-wake and temperature rhythms. In fact, most of the previous studies on circadian rhythm in dementia patients were done in their nursing home environment, with the exception of a few studies.
21,31 In our study, the clinical severity of AD patients was mild or moderate (CDR: 1 or 2), while AD patients seemed to have higher CDR scores in the previous studies of Satlin et al.
32 and Bliwise at al.
2 Mean MMSE scores (SD) of the subjects were 0.6 (1.1) and 8.9 (4.7), respectively. No significant difference in the values of circadian parameters between the AD and elderly comparison groups could be explained by a relatively less severe clinical state of dementia patients in our study. It has been reported that no change in the amplitude of sleep-wake cycle was observed in mild AD patients.
33 Witting et al.
34 also reported that the sleep-wake rhythm is disturbed in AD patients and correlates with the severity of dementia. To the contrary, a recent study didn't show any correlation between the two.
10 In our study, the number of AD patients was too small to conduct the correlation analysis between the amplitude of sleep-wake rhythm and the severity of dementia.
Both the skin temperature and sleep-wake rhythms are regulated by the “Y” pacemaker, which is a labile and weak circadian oscillator, while the core body temperature rhythm by the “X” pacemaker is stable and strong.
35 Therefore, the phase difference in our result has a different meaning from the phase relationship between the two major circadian oscillators, “X” and “Y.” In other words, the significant shortening of the phase difference between the two rhythms in our study does not actually indicate an internal desynchronization, which has been suggested as a possible biological marker of AD.
36 However, the change in the phase relationship between two different rhythms in AD patients, relative to that of the elderly comparison subjects, suggests that the stability of their relationship was broken to some degree. However, it will be difficult to exclude a false negative possibility of our finding because of its small sample size.
It has been noted that the sundowning syndrome in AD was closely related with the sleep-wake rhythm disturbance.
2,32 Our study showed no significant change in the absolute values of the circadian variables, but with the change in the circadian organization in AD patients relative to elderly comparison subjects, we could still address the significance of the phase difference in our study since it was shown even in AD patients without serious sleep or behavioral disturbances such as sundowning syndrome. As illustrated in
Figure 2, the variability in the skin temperature profile for 24 hours was remarkably higher, as compared to the core body temperature profile shown in previous studies.
37,38 However, skin temperature still showed a clear circadian organization in our study and would reflect change in the phase relationship with the sleep-wake rhythm. Furthermore, most of the subjects felt relatively comfortable with the skin temperature monitoring in our study.
In our study, the statistical analysis was conducted based on the frequently used method in previous studies on circadian rhythms. However, a slightly different analysis was added to a simple cosinor analysis of the sleep-wake rhythm. The Model 1 analysis did not show any significantly different result from that of the Model 2 analysis, except the reversed phase relationship between the two rhythms in the AD group. In the case of showing a distinct circadian organization, applying an alternative way of analysis for a certain circadian rhythm would be required.