A substantial proportion of schizophrenia patients (roughly 10% to 25%) also meet DSM-IV criteria for obsessive-compulsive disorder. Obsessive-compulsive disorder (OCD) in schizophrenia has been revealed in epidemiological studies and in clinical samples of adolescent, adult, and elderly schizophrenia patients.
1 In addition, it seems that obsessive-compulsive symptoms in schizophrenia may not be a result of chronic illness or neuroleptic treatment, since OCD has been found in first-episode, predominantly drug-naïve schizophrenia patients.
2,
3 The validity of the schizophrenia-OCD association is also supported by the outcome differences in schizophrenia patients with and without OCD, with schizo-obsessive individuals having poorer prognoses, greater impairment in functioning, and treatment resistance.
4 –
6 Schizophrenia and OCD are considered to be neurodevelopmental disorders with structural and functional brain abnormalities in the prefrontal cortex, striatum, and thalamus being consistently implicated in both disorders.
7,
8 There is growing evidence indicating that neurological soft signs are prevalent in schizophrenia patients and represent valid and reliable indicators of brain dysfunction in schizophrenia.
9 Neurological soft signs are considered to be an intrinsic feature of schizophrenia rather than a side effect of antipsychotic agents, since they are present in drug-naïve first-episode schizophrenia patients.
10 –
12 Neurological soft signs have been found in high-risk individuals, monozygotic twins discordant for schizophrenia, and first-degree relatives, supporting their role as trait markers for schizophrenia.
9,
13Although findings regarding neurological soft signs in OCD are scarce compared with schizophrenia, some studies found an increased rate of neurological soft signs in patients with OCD compared with healthy subjects.
14 –
16 Several groups revealed soft signs in OCD patients that were comparable to those seen in schizophrenia, including deficits in motor speed and sequencing, suggestive of common frontal-subcortical dysfunction in both disorders.
17 In the present study, we sought to evaluate the rate and the pattern of distribution of neurological soft signs in schizophrenia patients who also meet DSM-IV criteria for OCD (“schizo-obsessive”disorder). We assumed that neurological soft signs, as a neurobiological trait marker, may differentiate between the complex schizo-obsessive phenotype and “pure” schizophrenia.
We tested two alternative hypotheses: 1) the schizo-obsessive phenotype is associated with a unique pattern of neurological soft signs dissimilar to those exerted by non-OCD schizophrenia patients; 2) schizo-obsessive patients exhibit a higher rate of neurological soft signs than their non-OCD schizophrenia counterparts due to a “double jeopardy” of neurological deficits stemming from both schizophrenia and OCD components of the schizo-obsessive association.
METHOD
Subjects
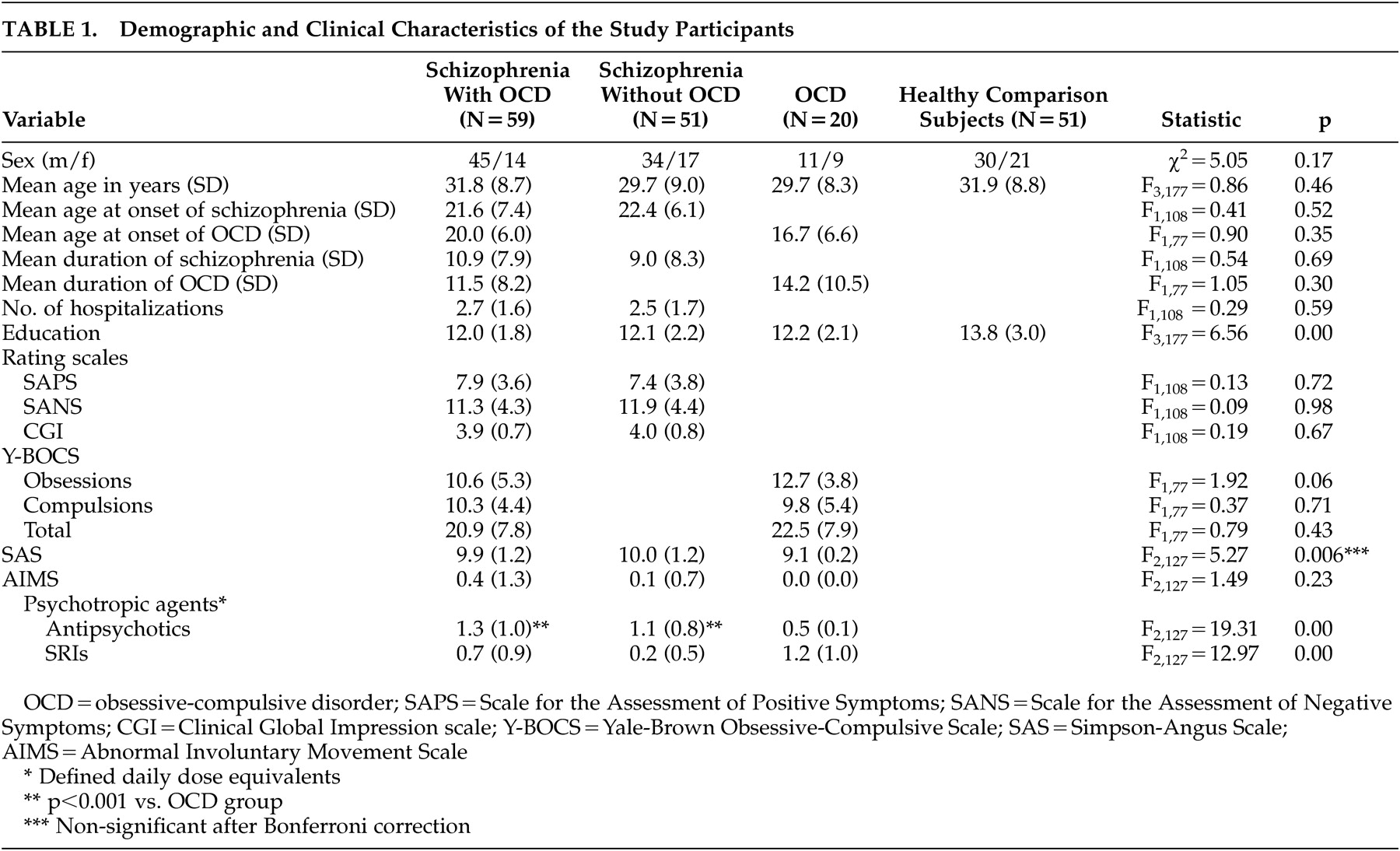
The study was conducted in Tirat Carmel Mental Health Center (Tirat Carmel, Israel). The study group included 59 patients who were admitted because of acute exacerbation of psychosis and who met DSM-IV criteria for both schizophrenia and OCD. Twenty (33.4%) of 59 participants in this group represent a subset of first-episode schizophrenia patients. Patients with affective and organic mental disorders and substance-induced psychoses were not included. Since antipsychotic agents, primarily clozapine, have been associated with the occurrence de novo and/or the exacerbation of OCD in schizophrenia patients,
1 patients with onset of OCD following the initiation of antipsychotic treatment were excluded. The major comparison group included 51 schizophrenia patients without OCD (first-episode: 16 of 51 [31.4%]), matched for age (SD=3 years) and number of hospitalizations to the schizo-obsessive group. We also recruited 20 nonschizophrenia OCD patients who were treated in the outpatient clinic of the Tirat Carmel Hospital to test the hypothesis regarding the “additive” effect of schizophrenia- and OCD-related neurological deficits in the schizo-obsessive group. This group included mostly treatment-resistant patients with moderate-to-severe obsessive-compulsive symptoms requiring combined treatment with serotonin reuptake inhibitors (SRIs) and antipsychotic agents (N=12). In addition, healthy comparison subjects (N=51) were recruited from among the hospital staff. Demographic and clinical characteristics of the study participants are shown in
Table 1 . The study was approved by the Institutional Review Board of Tirat Carmel Mental Health Center. All participants gave written informed consent after receiving a full explanation of the study protocol.
Clinical Assessments
The Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P),
18 was used for the diagnoses of schizophrenia and OCD. All participants underwent a direct clinical interview. Patients from the two schizophrenia groups were interviewed after resolution of acute psychosis when they were cooperative enough to undergo a structured clinical interview.
Severity of obsessive-compulsive symptoms was evaluated using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS).
19 To be included in the schizo-obsessive group, patients had to have typical obsessive-compulsive symptoms (i.e., time-consuming [≥1 hour], distressful obsessions and/or compulsions as ascertained by the Y-BOCS symptom checklist), which significantly interfered with a patient’s functioning. To be included in the schizo-obsessive group, we set the duration criterion for OCD at ≥6 months. If obsessive-compulsive symptoms were related to the content of delusions, hallucinations, or formal thought disorders, we required the presence of additional typical obsessive-compulsive symptoms (from the Y-BOCS checklist). Patients who exhibited obsessive-compulsive symptoms exclusively restricted to positive schizophrenia symptoms (e.g., compulsive handwashing due to command hallucinations) were excluded.
Neurological soft signs were assessed using the Neurological Evaluation Scale (NES),
20 a structured scale presenting scores in subscales of motor coordination, sensory integration, sequencing of complex motor acts and “others” signs. There are 26 items rated on a scale of 0 to 2 (0=normal, 1=some disruption, 2=major disruption), according to standardized instructions. The motor coordination subscale includes tandem walk, rapid alternation movements, finger/thumb opposition, and the finger-to-nose test. The sensory integration subscale includes audiovisual integration, stereognosis, graphesthesia, extinction, and right/left confusion. Sequencing of motor acts includes the first-ring test, the first-edge-palm test, the Ozeretski test, and rhythm-taping test. The “others” signs include short-term memory, eye movements abnormalities, frontal release signs, and primitive reflexes.
Severity of schizophrenia symptoms was assessed with the Scale for the Assessment of Positive Symptoms
21 (SAPS) and the Scale for the Assessment of Negative Symptoms
22 (SANS), and the Clinical Global Impression (CGI) scale was used for psychosis.
23Antipsychotic-induced extrapyramidal side effects were assessed using the Simpson-Angus Scale
24 (SAS), and tardive dyskinesia, using the Abnormal Involuntary Movement Scale
23 (AIMS). Patients’ medications were recorded, and dosages for all psychotropic drugs were converted to defined daily dosage (average maintenance dosage), as determined by WHO Collaborating Center for Drug Statistics Methodology.
25 All patients in the two schizophrenia groups were treated by antipsychotic agents: risperidone (schizo-obsessive: 10 patients; schizophrenia: eight patients), olanzapine (19 and 16 patients, respectively), quetiapine (three and two patients), clozapine (14 and eight patients), haloperidol (nine and 11 patients), and perphenazine (six and six patients). Twenty-seven schizo-obsessive patients and seven schizophrenia patients were treated by SRIs.
The NES was administered by a senior psychiatrist (AP) who was extensively trained in neurological evaluation and who was blind to the patients’ group assignment.
All clinical interviews were conducted by a trained psychologist (SF) who has extensive experience in the clinical evaluation of patients with schizophrenia, schizo-obsessive disorder and OCD.
Statistical Analysis
All statistical analyses were performed using SPSS, version 12 (SPSS Inc., Chicago, III). Between-group differences in demographic and clinical characteristics were compared using analysis of variance (ANOVA) and the chi-square test, as appropriate. Group differences in the NES subscale scores were examined using one- or two-way ANOVA, as appropriate, followed by Bonferroni’s multiple comparison procedure when significant main effects were present. Pearson’s correlation tests between the NES subscale scores and clinical rating scale scores (SAPS, SANS, CGI), extrapyramidal side effects (SAS, AIMS) and defined daily dosage of psychotropic agents were also used. A p value of <0.05, two tailed, was considered significant.
RESULTS
Demographic and clinical characteristics of the study participants are shown in the
Table 1 . There was no significant difference in the proportion of men and women or the mean age of the study participants in the four groups. The healthy comparison subjects had significantly more years of education than patients (
Table 1 ).
Patients in the two schizophrenia groups (with and without OCD) were remarkably similar in demographic and clinical characteristics and clinical rating scale scores (
Table 1 ), as well as dosages of antipsychotic agents, as reflected by defined daily dosages (p>0.05). The schizo-obsessive patients had significantly higher dosages of SRIs (p<0.01) (
Table 1 ).
The schizo-obsessive and the “pure” OCD group did not differ significantly in the age of onset, duration and severity of OCD symptoms, as reflected by the Y-BOCS scores and dosages of SRIs (
Table 1 ). The schizo-obsessive patients had significantly higher dosages of antipsychotic agents (p<0.05).
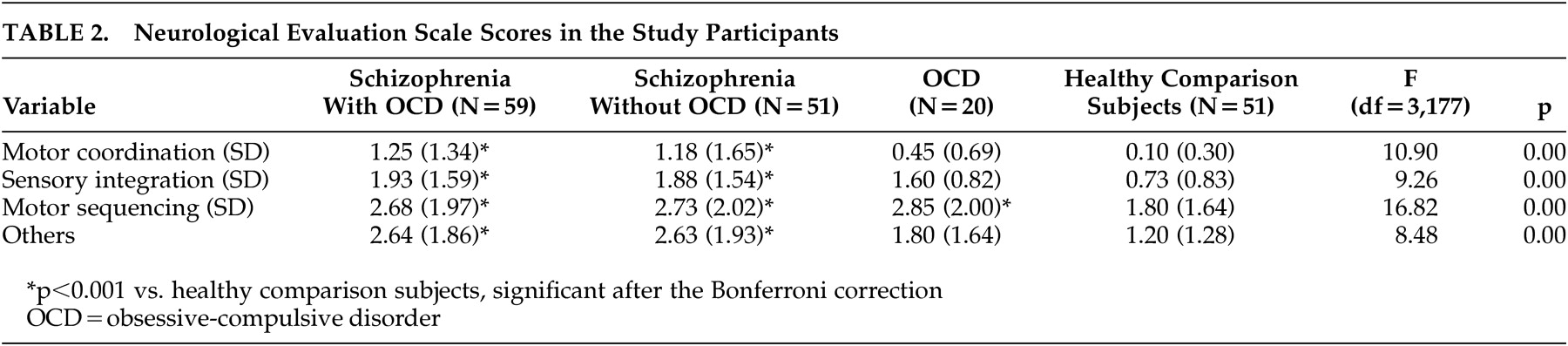
Defining neurological impairment as the presence of at least one neurological sign (a score of 2 on the NES), 54% (32/59) in the schizo-obsessive group, 37% (19/51) in the schizophrenia group, 40% (8/20) in the OCD group and only 5.9% (3/51) in the comparison group met this criterion (p<0.001, each patient group versus comparison subjects), with no significant difference between the two schizophrenia groups (chi-square=3.17, p=0.07). ANOVA revealed a significant main effect of the group on all NES subscale scores, with patients in the two schizophrenia groups having substantially higher scores than the healthy comparison subjects (
Table 2 ).
Comparison between the two schizophrenia groups (with and without OCD) did not reveal any differences on the NES subscales (p=1.00 for all NES subscales). There were no differences in any of the NES subscales between the subsets of first-episode patients in the two schizophrenia groups (F 1,105 =3.20 to 0.15, p=0.08 to 0.70).
It is of note that within the schizo-obsessive group the first-episode patients did not differ significantly from patients with repeated hospitalizations on any of the NES subscale scores (motor coordination: 1.3 [SD=1.6] versus 1.3 [SD=1.3]; F 1,56 =0.002, p=0.96; sensory integration: 2.2 [SD=2.0] versus 1.8 [SD=1.4]; F 1,56 =0.86, p=0.36; motor sequencing: 2.4 [SD=1.9] versus 2.7 [SD=2.0]; F 1,56 =0.48, p=0.49; “others”: 3.2 [SD=2.4] versus 2.4 [SD=1.5]; F 1,56 =2.06, p=0.16). Similarly, no significant difference on the NES was found between the subset of first- and repeated-episode patients in the non-OCD schizophrenia group (p>0.05 for all NES subscales).
No correlation was found between the NES subscale scores and any of the demographic variables, clinical rating scales, extrapyramidal side effects measures, and defined daily dosage of psychotropic agents (r=0.02–0.23, p>0.05).
Interestingly, the OCD patients scored similarly to the two schizophrenia groups and significantly higher than the healthy comparison subjects on the NES motor sequencing subscale (OCD versus comparison subjects, p<0.01) (
Table 2 ).
DISCUSSION
The major finding of this study is that soft neurological signs, as assessed by the NES, did not differentiate between schizophrenia patients with and without OCD. In contrast to our hypothesis, patients in the two schizophrenia groups, regardless of the presence of OCD, had a substantial and remarkably similar “soft neurological deficit” in motor and sensory areas, as compared with healthy individuals. It is unlikely that a relatively small sample size of the two schizophrenia groups accounted for not detecting the between-group difference (type II error), since not even a minor signal indicating a possible group difference was noted. It is also implausible that higher dosages of antiobsessive agents in the schizo-obsessive group play a role, since SRIs seem not to have an effect on neurological soft signs; however, direct evaluation of their impact on neurological soft signs is warranted. Our findings accord with a recent comparative study of a smaller sample size evaluating soft neurological signs using NES in schizophrenia patients with and without OCD, which also failed to find any significant group differences.
26 Hence, it is conceivable that a pervasive schizophrenia-related “soft neurological deficit” superimposes any additional deficits related to a comorbid disorder, such as OCD. Our findings may also suggest that rather than representing a simple comorbidity between the two disorders and showing a combined neurological impairment, the schizophrenia-OCD association, at least from the perspective of the revealed neurological deficit, is a part of the schizophrenia spectrum disorders. Evaluation of neurological soft signs in larger samples of schizo-obsessive and non-OCD schizophrenia patients, in conjunction with neurocognitive and neuroimaging techniques, may contribute to the detection of common and specific neurological deficits in subsets of schizophrenia patients with and without OCD.
Notably, in both groups a subgroup of first-episode schizophrenia patients did not vary from the patients with repeated hospitalizations in the rate and the pattern of neurological soft signs. These findings support growing evidence indicating that neurological soft signs are biological trait markers, detectable early in the illness.
27,
28 In addition, since no correlation was found between neurological soft signs and psychopathological variables, extrapyramidal side effect scores or defined daily dosage for antipsychotic agents in both schizophrenia groups, our findings point to the independent nature of neurological soft signs in schizophrenia.
Although this study was not powered to compare neurological soft signs in schizophrenia and OCD patients, some preliminary comments regarding a neurological deficit in OCD are noteworthy. The OCD patients showed a substantial deficit in the NES motor sequencing subscale comparable to both schizophrenia groups. Bolton et al.
17 also showed a similarity between schizophrenia and OCD patients on the sensory integration and complex motor task domains. In contrast, their schizophrenia patients, compared to the OCD group, exhibited more neurological soft signs in motor coordination and “hard signs.” Poor performance in complex motor sequencing tasks may result from a dysfunction of the frontal-basal ganglia circuitry in both schizophrenia and OCD.
9,
11 It is plausible that although neurological soft signs are considered to be nonlocalized neurological abnormalities, they do reflect dysfunctions in specific brain circuits implicated in both disorders. It should be noted that our OCD group included mostly treatment-resistant patients with moderate to severe obsessive-compulsive symptoms. When examining severe forms of illnesses, it is less likely that between-group differences on soft neurological signs will be detected.
In conclusion, the results of this study indicate that soft neurological signs do not discriminate between schizophrenia patients with and without OCD and seem to be of limited value as a putative endophenotype in a search for specific etiological mechanisms underlying a schizo-obsessive subgroup of schizophrenia. Further search for alternative neurobiological markers (cognitive, neuroimaging) is warranted to clarify the delineation of a specific schizo-obsessive clinical phenotype.
Acknowledgments
The authors thank Rena Kurs for her assistance in preparation of the manuscript.