I n order to meet diagnostic criteria for chronic posttraumatic stress disorder (PTSD), traumatized individuals must report significant problems with reexperiencing symptoms, avoidance symptoms, and arousal symptoms. Included in the diagnostic criteria for avoidance is a subjective restriction in affective range. While not absolutely necessary for the diagnosis, a restricted range of affect is a common complaint among veteran patients with chronic PTSD, and is frequently encountered in combination with feelings of detachment from others and diminished interest in significant activities. This subjective reduction in affective experience has both qualitative and quantitative aspects. Patients with PTSD frequently report an overall decrease in emotional experience, but may report a significant, more selective reduction or complete absence of “warm” emotions such as empathy.
1 To date, researchers have examined the responses of patients with PTSD to affective stimuli, but the primary goal of these studies has been on evaluating potentially threatening stimuli, such as angry faces
2 or to trauma reminders.
3 None have examined these patients’ ability to comprehend affective stimuli associated with verbal communication. While research attention to threatening affective stimuli in patients with PTSD is obviously warranted, few studies have attempted to determine if overall emotional perception is impaired in patients with chronic PTSD. An inability to correctly interpret the nuances of affective expression in others has been shown to be present in other chronic mental disturbances, such as schizophrenia.
4,
36In order to examine affective processing in patients with chronic PTSD, we used the Aprosodia Battery to assess these patients’ ability to comprehend and discriminate the affective aspects of language and communication.
5 The Aprosodia Battery was developed to specifically distinguish between profiles of affective prosodic deficits caused by focal left brain damage versus focal right brain damage.
5,
6 Reducing verbal-articulatory demands in patients with left brain damage improves their performance on the Aprosodia Battery, whereas reducing verbal-articulatory demands in patients with right brain damage does not improve their performance (see Methods, Results, and
Figure 1 for more details). Comprehension of affective prosody appears to be a lateralized and dominant function of the right hemisphere with the posterior Sylvian region serving as the nodal point of a distributed parallel network for comprehension, similar to Wernicke’s area in the left hemisphere serving as the nodal point for comprehension of the verbal-linguistic aspects of communication.
6 In order to examine the ability of PTSD subjects to comprehend affective prosody, we evaluated their performance relative to a group of healthy comparison subjects and a group of individuals with focal ischemic infarctions involving either the right or left hemisphere.
METHODS
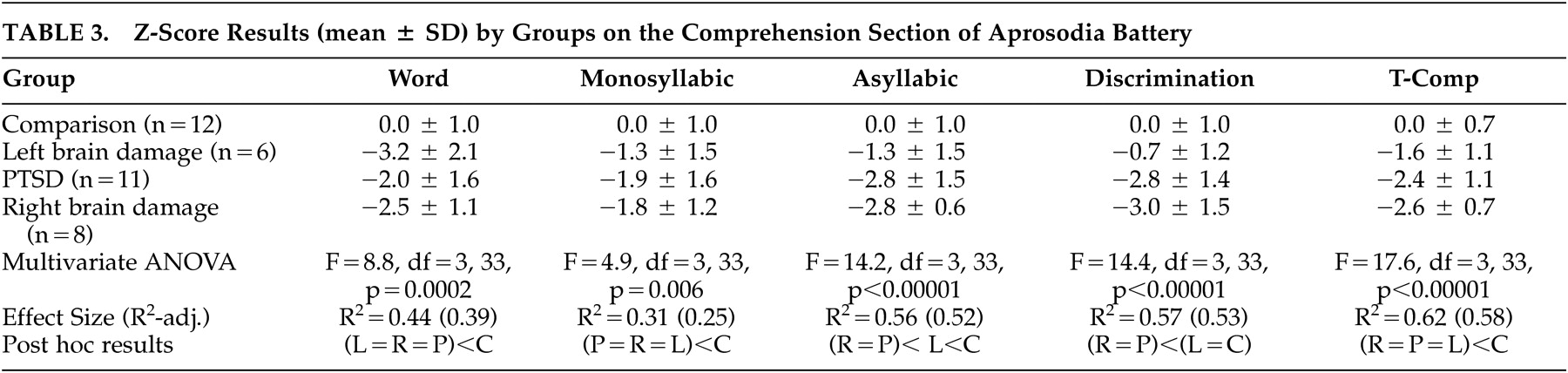
The study involved four research groups. Individuals with PTSD were recruited through the North Little Rock Veterans Association (VA) as part of an ongoing study of affective processing in PTSD. The data for the age-matched healthy comparison subjects and left brain damage and right brain damage comparison groups were obtained from the Affective Communication Research Laboratory database at the Oklahoma City VA as part of a long-term study of the neurology of affective prosody that was initiated in Fargo, N.D.
4 –
12 All participants were recruited under institutional review board protocols through either the VA Hospitals in North Little Rock, Ark., Oklahoma City, or Fargo; the Merit Care Hospital in Fargo, N.D.; or the University of Oklahoma Health Sciences Center Hospitals in Oklahoma City. Informed consent was obtained in keeping with the Declaration of Helsinki. If a patient was unable to give informed consent because of aphasia or loss of insight into their clinical condition, consent was sought from their next of kin or legal guardian. The demographics of the research groups are summarized in
Table 1 . All subjects in the PTSD group were receiving service-connected disability for PTSD. The PTSD group was all male while the other groups had male and female subjects. Using multiple Fisher’s exact test, no sex differences were found between the non-PTSD groups (p>0.3 in all cases), but all non-PTSD groups differed from the PTSD group (p≤0.05 in all cases). It should be noted, however, that previous research using the Aprosodia Battery has not yet uncovered either a sex or educational effect for any of the Comprehension subtests.
6 –
8,
11,
12 The only sex effect observed, which is not pertinent to this article, is that elderly men perform marginally better on Asyllabic Repetition compared with women.
The primary research group consisted of 11 individuals with Vietnam era, combat-related PTSD. The diagnosis of PTSD was made using the most recent version of the Clinician Administered PTSD Scale (CAPS-2), a structured interview for the assessment of PTSD symptoms.
13 The Structured Clinical Interview for DSM-IV (SCID)
14 was also administered to all patients with PTSD, and was used to assess for the presence of axis I psychiatric illness. The patients with PTSD were excluded if they had a history of neurological disease, including head injury with any loss of consciousness, stroke, or neurodegenerative disorders. All participants met both SCID and CAPS-2 criteria for current and lifetime PTSD. The patients with PTSD were also assessed for histories of alcoholism using the Michigan Alcoholism Screening Test (MAST), Obsessive Compulsive Drinking Scale (OCDS),
15 and questions regarding years of alcohol use, because alcohol usage, especially early exposure as a teenager, has been shown to be a factor in performance on the Aprosodia Battery.
9,
10 If alcohol abuse is initiated as a young adult (20+ years old), it has little, if any, effect on the production or comprehension of affective prosody.
From the database of the Affective Communication Research Laboratory, appropriate subjects were identified who were age-matched to the PTSD group and included 12 healthy comparison subjects, seven patients with left brain damage, and nine patients with right brain damage. All patients with left and right brain damage had focal ischemic infarctions that predominantly involved cortex and adjacent white matter by MRI scan. All participants were native speakers of English, strongly right-handed based on self or family report and scored ≥0.70 on the Edinburgh Handedness Scale.
16 The non-PTSD subjects were screened for comorbid medical, neurological, and psychiatric illnesses that may be associated with cognitive decline or alterations in affect. Particpants were excluded if they had major psychiatric illness (e.g., schizophrenia, bipolar disorder), severe medical conditions (e.g., uncontrolled diabetes or congestive heart, pulmonary, renal, or liver failure), alcoholism, or neurological conditions affecting the CNS (e.g., head trauma with residual deficits, dementia or other neurodegenerative disorders). Individuals were also excluded if they were taking medications that could interfere with testing, such as neuroleptics, high-dose beta-blockers, or benzodiazepines. None of the patients had either histories or evidence on MRI scan of a previous stroke. Testing of the brain-damaged patients was completed within 3 to 8 weeks poststroke. This was done to avoid the confounding effects of acute and potentially reversible pathophysiologic processes, such as diaschisis, ischemic penumbra or edema,
5,
6,
17 –
19 and long-term improvement from spontaneous recovery due to neural reorganization.
5,
6,
20,
21Aprosodia Battery
The Aprosodia Battery was developed to distinguish patterns of deficits related to right versus left focal brain damage as the result of ischemic injury.
5,
6 It has been used as an effective research tool in other clinical populations, including subjects with schizophrenia,
4 alcohol exposure and abuse,
9,
10 and Alzheimer’s disease,
7 producing highly robust results. The Aprosodia Battery consists of two parts, Production and Comprehension. Only the Comprehension portion was administered to the PTSD group that includes three identification tasks (word, monosyllabic, and asyllabic) and a discrimination task. The names of the identification tasks indicate the type of utterance used to carry the affective-prosodic stimuli.
Each identification task consists of 12 utterances, two renditions each of six emotions (happy, sad, disinterested, neutral, surprised, and angry), with one rendition having emphatic stress early in the utterance and the other rendition having emphatic stress late in the utterance. The 12 stimuli are presented twice in randomized order for 24 exemplars per task. Subjects are asked to identify the emotional intonation of each stimulus by choosing the appropriate affect from a vertical array of six line drawings of faces expressing different affects. Next to each face is the corresponding written label of “neutral,” “happy,” “sad,” “disinterested,” “surprised,” and “angry.” On the discrimination task, the subjects indicate if a pair of stimuli has the same or different emotions. The stimuli are the same as those used for word identification, but they have undergone low-pass filtering at 300 Hz, (Krohn-Hite Variable filter, model 3550). This procedure preserves prosodic-acoustic information involving intonation and intensity, both globally (affective) and locally (stress), while degrading phonetic information.
22,
23 Twenty-four pairs of stimuli were used. Twelve pairs had the same affective intonation with different stress patterns and 12 had different intonations with the same stress pattern. For each pair of stimuli, participants were asked to indicate whether the pair had the same or different emotions. If participants base their answers on stress rather than intonation information, they will perform poorly on this task.
The stimuli were played to the participants in a quiet room using either a tape recorder or CD player with speakers whose volume was set to a level that was audible for the subject. Previous research has shown that this is an acceptable technique, even in patients who have moderate hearing loss that does not require the use of hearing aides.
11Statistical Analysis
The results were analyzed statistically with SPSS 8.0 for Windows, using multivariate, repeated-measures analysis of variance (ANOVA) and linear regression analyses with significance set at 0.05 for main effects and interactions. Post-hoc relationships were determined using Student-Newman-Kuels analyses. Effect sizes, based on measures of the strength of association derived from correlation indices, indicate how well the experimental variables explain the variance in the data. They are given as partial
eta 2 (
p η
2 ) for repeated-measure ANOVAs and as R
2 and R
2 -adjusted for multivariate ANOVAs.
24 –
27 For nonadjusted strength of association statistics (η
2, r
2, R
2 ), results range from 0 (0%) or no association to 1 (100%) or complete association. R
2 -adjusted is a more conservative strength of association measure than R
2 because it because it takes into account degrees of freedom when it is calculated. Occasionally, R
2 -adjusted may be a small negative number. When this occurs, the statistical interpretation is that no association exists. As reported by SPSS, the η
2 values for repeated-measures ANOVAs are actually
p η
2, which is a special statistic used in complex factorial designs to gauge the strength of relationship for a given effect while holding others constant.
28 It is considered a better estimate of effect size for the effect being evaluated than η
2 .
24,
29 For behavioral research, correlation indices between 0.1 to 0.3 are considered small effects, values between 0.3 and 0.5 are considered medium effects, and values of >0.5 are considered large effects. Respective squared-values measuring the strength of association equal 0.01 to 0.09 for small, 0.09 to 0.25 for medium, and >0.25 for large effect sizes.
25,
30 None of the statistical analyses detected violation of homogeneity of variance based on the Levene’s test (p>0.01 in all cases).
RESULTS
Affective Prosody Battery
The Z-scores for all subjects were calculated for each comprehension task based on the performance of comparison subjects using the following formula: [(Subject score−comparison mean score)/comparison SD].
31 This data transformation does not alter statistical relations but removes any variability in performance across the comprehension tasks of the Aprosodia Battery attributable to comparison subjects, leaving intact the residual variability attributable to patient groups so that if statistical interactions are found they may be more easily understood.
4 –
6 If any score of the four Comprehension tasks was <−1.64, then Comprehension was considered impaired in that subject.
5,
6 A Z-score of ≥−1.64 represents the expected performance for 95% of comparison subjects.
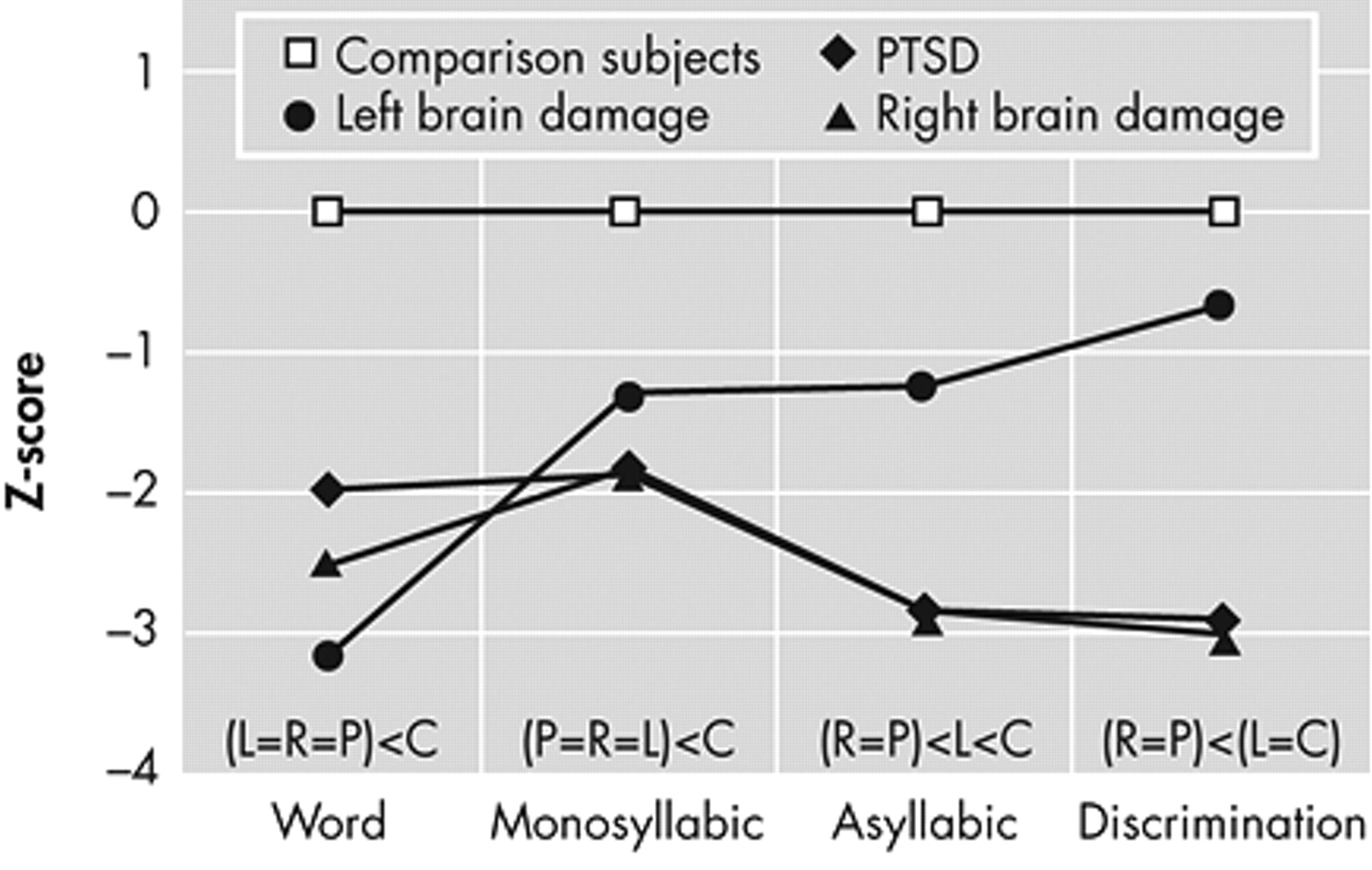
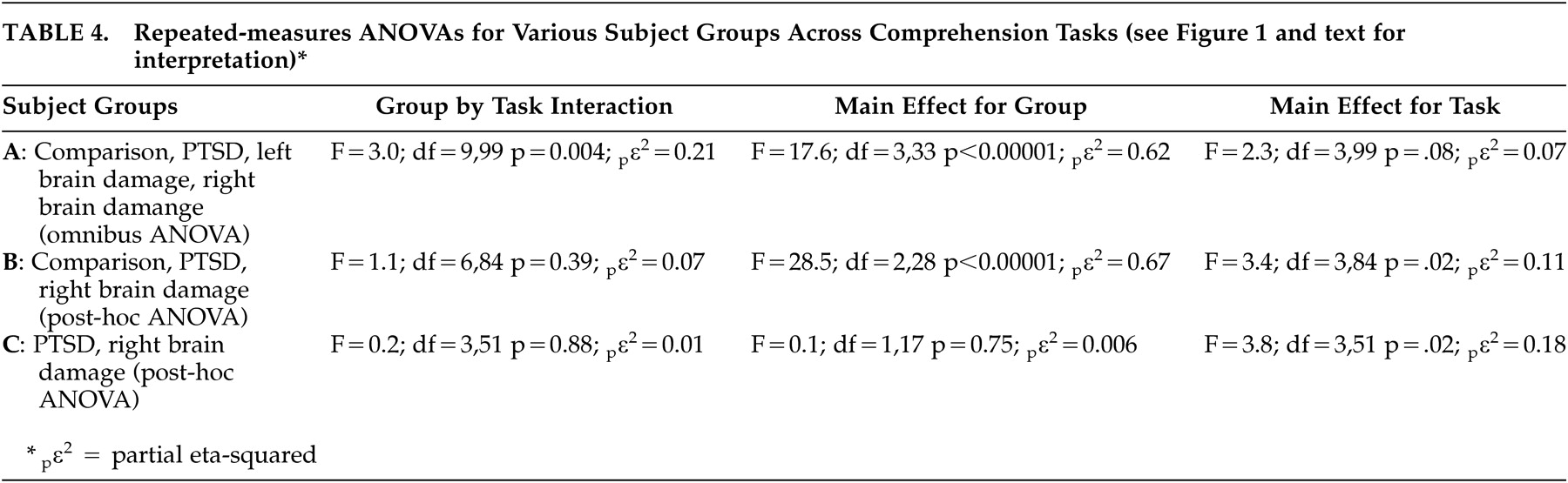
31 Using this criterion for establishing abnormal performance, all 11 PTSD patients were impaired and six of seven patients with left brain damage and eight of nine patients with right brain damage were impaired. Lastly, a total comprehension score (T-Comp) was calculated for each subject by averaging the results on the four Comprehension tasks. The data then underwent a multivariate ANOVA with post hoc analyses to determine statistical relationships among groups for each task. The group means, standard deviations, and results of the statistical analyses are presented in
Table 3 .
To better assess statistical interactions across the Comprehension tasks, the data underwent an omnibus repeated-measure ANOVA with subject groups as the independent variables and the Z-score results for the three identification tasks and the discrimination task as the dependent variables (see
Figure 1 and
Table 4 ). There was a significant group by task interaction (F=3.0, df=9, 99, p=0.004;
p η
2 =0.21) and a highly robust main effect for group (F=17.6, df=3, 33, p<0.00001;
p η
2 =0.62). The main effect for task was not significant (F=2.3, df=3, 99, p=0.08;
p η
2 =0.07) but showed a small effect size. On inspecting
Figure 1, two observations are apparent: the interaction appears to be the result of improvement across tasks by the left brain damage group, and the performance of the right brain damage and PTSD groups appears identical. To test these observations statistically, we performed a post-hoc repeated-measure ANOVA that only included the healthy comparison group, the patients with right brain damage, and the PTSD groups as independent variables. The results (
Table 4 ) revealed a nonsignificant group by task interaction (p=0.39;
p η
2 =0.07) with a robust main effect for groups (p<0.00001;
p η
2 =0.67) and a significant main effect for task (p=0.02;
p η
2 =0.11). Thus, the group by task interaction observed in the original omnibus ANOVA is due to the left brain damage group’s performance. Finally, a second post-hoc repeated-measure ANOVA was done that only included the right brain damage and PTSD groups as independent variables. The results (
Table 4 ) demonstrated a nonsignificant group by task interaction (p=0.88;
p η
2 =0.01) and a nonsignificant main effect for groups (p=0.75;
p η
2 =0.006) indicating that the performance of the right brain damage and PTSD across tasks was identical statistically. The was a significant main effect for task (p=0.02;
p η
2 =0.18) because of the overall worsening of performance on the Asyllabic and Discrimination tasks compared to the word and monosyllabic tasks in both the right brain damage and PTSD groups (
Figure 1 ;
Table 3 ).
Relationship of Alcohol Use/Abuse to Performance on the Aprosodia Battery
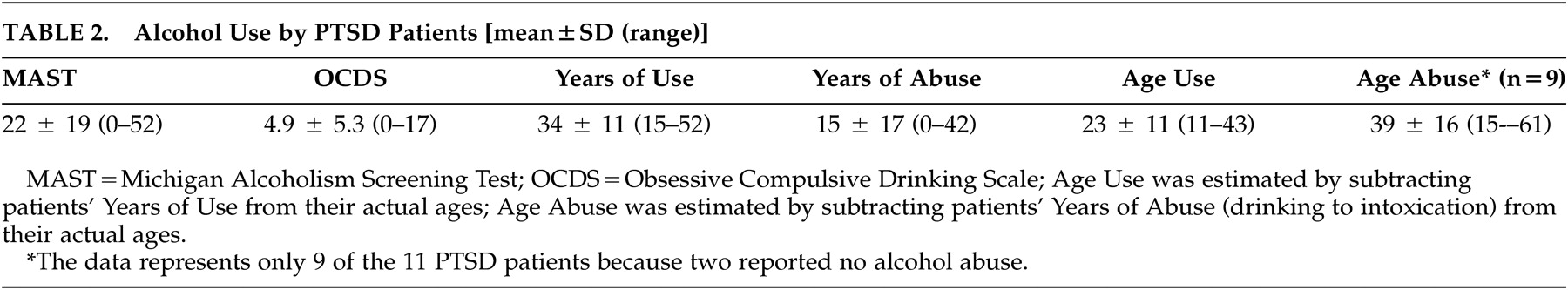
Lastly, multiple stepwise linear regression analyses were run to assess if any of the alcohol use/abuse indicators (
Table 2 ) in the PTSD group predicted performance on the comprehension subtests of the Aprosodia Battery. Probability of F to enter was set at ≤0.05 and probability of F to remove was set at ≥0.10. None of the alcohol use/abuse indicators predicted performance on any of the comprehension subtests. This result is consistent with previous research showing that alcohol abuse starting in adulthood (20+ years old), after the brain is fully matured, is not associated with deficits in Comprehension of affective prosody.
9 Nine of 11 PTSD patients in our sample had a history of alcohol abuse; eight subjects started their abuse between 24 and 61 years old and only one subject began his abuse at 15 years old. Therefore, it is unlikely that the affective-prosodic comprehension deficits found in our PTSD group can attributed to alcohol use/abuse.
DISCUSSION
Compared to healthy comparison subjects, veterans with chronic PTSD performed poorly on the Aprosodia Battery, on a par with patients with right hemisphere brain damage. This finding has implications for the treatment of veterans with chronic PTSD, and suggests avenues for further research into this condition.
The poor performance of veterans with chronic PTSD on the Aprosodia Battery is not likely to be surprising to clinicians familiar with this population. Families of patients with chronic PTSD frequently complain to clinicians that their affected family member seems to be impaired in reading emotions of others, or that they are being ignored or misperceived by their affected family member. This misperception can lead to frustration and anger on the part of PTSD patients, as well as the family, and may worsen psychosocial adjustment among patients with PTSD.
While affective prosodic deficits in patients with PTSD have been the subject of limited study, the similar concept of alexithymia has been examined in this population.
32 Alexithymia means literally, “no words for affect” and in some respects resembles aprosodic deficits observed after brain damage. However, alexithymia describes a completely subjective experience of impoverished imagination and limited ability to verbally describe feelings, whereas aprosodia refers to a testable limitation in the perception and expression of affect associated with language and communication, in which internal feeling states are usually preserved.
37 –
39The ability to accurately interpret the affective components of verbal communication is an important skill; impairments in this ability could lead to disruptions in social function and worsen overall outcomes for patients with chronic PTSD, as they do in patients with schizophrenia.
33,
36 Of interest, patients with schizophrenia show a pattern of comprehension deficits on the Aprosodia Battery that is indistinguishable from patients with right focal brain lesions
4 and, thus, with the PTSD patients reported here. The treatment of veterans with chronic PTSD typically combines pharmacotheraputic and psychotherapeutic approaches; impairments in affective comprehension could potentially interfere with participation in psychotherapy. This restricted ability could conceivably contribute to the overall poor response of some veteran PTSD patients to structured psychotherapeutic interventions.
In the absence of physical brain injury, it is difficult to conceive neurophysiologically that a deficit in affective prosodic comprehension commensurate with individuals suffering from ischemic right cerebral brain damage could be acquired as a result of exposure to combat. Another hypothesis is that veterans with acquired PTSD may have had preexisting affective prosodic deficits that make them more vulnerable to acquiring PTSD when exposed to combat, perhaps as part of a right hemisphere developmental “learning disability.”
34,
35 Another possibility is that they may have had toxic exposure to ethanol in utero that is not sufficient to cause either fetal alcohol syndrome or fetal alcohol effects,
9 especially since families of patients with PTSD often have significant histories of alcohol abuse and dependence.
40 However, sorting out the possible etiologies of the prosodic deficits found in these PTSD patients is clearly beyond the scope of this study. Further research should be directed at determining more about overall affective processing, both pre and postmorbidly, in subjects with combat-related PTSD and exploring potentially important relationships between affective prosodic deficits, self-reports of alexithymia, and overall affective processing. Continued investigation into impaired affective processing in veterans with PTSD also offers the possibility of improving psychosocial interventions for this disabled population.