W hat are the neural correlates of anxiety in healthy adult brains? In this study we use an automated MRI technique to investigate the relationship between brain structure and self-report measures of level of anxiety on a voxel-by-voxel basis. Anxiety may be characterized by a combination of physiological, behavioral, and cognitive components.
1 A distinction can be made between nonpathological anxiety that is found in the normal healthy population and anxiety disorders that require clinical intervention.
2 Dimensions of anxiety in a population of healthy subjects can be measured by the State-Trait Anxiety Inventory (STAI).
3 Trait anxiety is described as a relatively stable dimension of personality, while state anxiety is the predisposition to anxiety fluctuation.
Some researchers have argued that normal anxiety and anxiety disorders lie on a continuum where the boundary between normal and pathologic is arbitrary, as various cognitive biases are typically associated with anxiety in both healthy and clinical populations.
4 Therefore, trait and state anxiety provide a reasonable model for anxiety in human subjects and have been used as such in studies exploring cognition in the presence of anxiety. Previous PET, functional MRI, and magnetic resonance spectroscopy studies have investigated the neurobiology of “physiological” anxiety.
5 –
7 The aim of the present study is to evaluate the neural correlates of trait and state anxiety in a healthy population, focusing on brain regional structural characteristics rather than metabolism and function.
In the present study we applied voxel-based morphometry (VBM) to high-resolution structural MRI images
11 to assess the relationship between gray matter interindividual differences in volume and state-trait anxiety, as measured by the STAI. Although very little is known about the correlation between behavioral anxiety measurements and regional brain volume in healthy individuals,
18 based on previous structural MRI studies of anxiety disorders
12 –
17 our hypothesis was that there is an inverse correlation between morphometry of the neuroanatomical network of anxiety described in animal and clinical studies (including the hippocampus, amygdala, and PFC) and self-reported levels of anxiety.
RESULTS
The mean STAI-S score was 29.4 (SD=9.1) and the mean STAI-T score 29.1 (SD=6.83). Results on each scale were not statistically different between genders (STAI-S: two-tailed t test for equality of means, t=0.918, df=28; p=0.367; STAI-T: two-tailed t test for equality of means, t=1.025, df=28, p=0.314) and not different from normative data on healthy subjects.
3 Measures of total gray matter volume were not correlated either with STAI-S (Pearson correlation coefficient, two-tailed, r=−0.204, p=0.280) or with STAI-T (Pearson correlation coefficient, two-tailed, r=−0.135; p=0.477).
The Good et al.
20 optimized procedure was followed in order to obtain gray matter partitions for each subject. The resulting smoothed modulated normalized gray matter images were entered into multiple regression analyses using SPM2 to determine the brain regions whose volume varied with the anxiety measures across the participants. In addition to STAI-T and STAI-S measures, total gray matter volumes were entered into the multiple regression analysis in SPM2, in order to control for global differences in gray matter that could bias the correct interpretation of regional gray matter morphometry, as previously discussed.
A conjunction analysis was performed to detect morphometric changes in gray matter which were correlated with trait and state anxiety measures. The significance threshold for results was set at <0.001 (not corrected for multiple comparisons) and, in order to exclude small clusters, an extent threshold of 50 contiguous voxels was chosen. In VBM a statistical threshold of p<0.001 not corrected for multiple comparisons can be used when an
a priori hypothesis has been defined according to Ashburner et al.
22The multiple regression analysis revealed that in several brain regions gray matter volume had an inverse correlation with anxiety ratings (
Table 1 and
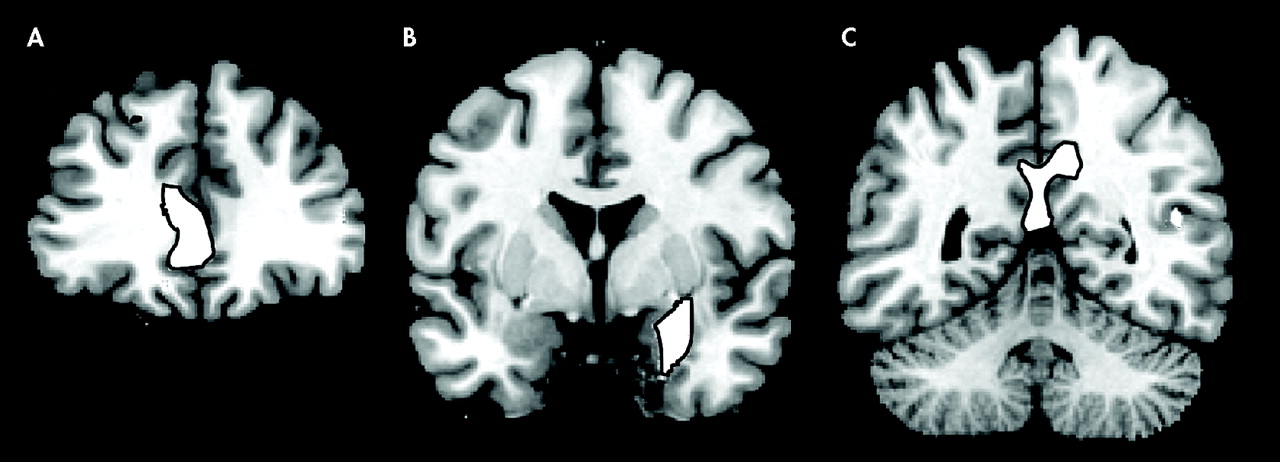
Figure 1 ). Significant negative correlations have been observed in the prefrontal cortex, in the dorsolateral PFC bilaterally and in the rostral divisions of anterior cingulate gyrus. In the posterior cortex, gray matter volume in the posterior and restrosplenial cingulate cortex showed a negative correlation with STAI-S and STAI-T. Another cluster displaying an inverse correlation included the left parahippocampal gyrus and the left amygdala.
In the absence of a well-defined
a priori hypothesis, we examined in an exploratory fashion positive correlations between gray matter volumes and STAI-S/STAI-T. Positive correlations were observed only between anxiety scores and regions of the ventrolateral PFC bilaterally (
Table 2 ).
DISCUSSION
Our findings show that a distributed neural network, including the parahippocampal-amygdalar regions, the posterior cingulate cortex, and medial and dorsolateral PFC, shows an inverse volumetric correlation with levels of reported anxiety in healthy volunteers. Subjects who had lower gray matter volume in certain areas of the mesial temporal lobe, posterior cingulate cortex, and medial and dorsolateral PFC tended to have higher levels of anxiety as measured using the STAI, while participants who had larger gray matter volume in the same regions had lower levels of anxiety.
A decreased volume of the mesial temporal lobe, including the parahippocampal-amygdalar region is an anatomical hallmark of anxiety disorders.
13,
14,
16,
23 Posttraumatic stress disorder (PTSD) has been associated with reduced hippocampal volumes or function,
12,
13,
15 –
17,
24 and panic disorder has been associated with structural and functional abnormalities in the parahippocampal gyri and amygdala.
25,
26 Decreased anterior cingulate volume was observed in patients with PTSD using manual tracing volumetric methods and VBM.
27,
28 Using the technique of VBM, a reduction in left parahippocampal gray matter concentration has been demonstrated in panic disorder patients.
29The role of the PFC in the expression of fear and anxiety has been previously investigated. Impaired PFC cognitive control over threatening information has been associated with elevated levels of reported and observed anxiety.
32 In addition, the rostral anterior cingulate cortex (ACC) control mechanisms over threat-related distracters may be weakened in anxiety.
6 In the human literature, structural changes in the pre- and subgenual divisions of the ACC have been demonstrated in women affected by PTSD.
33 In animal research, medial PFC has been associated with emotion-related behavior and inhibition of autonomic and defensive reactions.
34 Functional neuroimaging studies have demonstrated an inverse correlation between medial prefrontal cortex activation and PTSD symptom severity.
35 –
37 As a component of a neural network of anxiety, dysfunction of the medial prefrontal cortex would cause perseveration of conditioned fear response even when the threat level has been diminished.
38 Therefore the amygdala is responsible for assessing the emotional valence of stimuli, while the medial PFC adjusts the state of vigilance to the level of threat.
39The anterior regions of the ACC are involved in the regulation of emotion, while the posterior cingulate cortex (Brodmann’s areas 23 and 31) in the evaluation of the environment, and visuospatial or personal orientation.
38 Despite this dissociation, research studies on animals have shown a strong functional connection between the anterior and posterior cingulate cortices and likely both areas contribute to emotion processing, simple animal phobias and PTSD.
40,
41,
42 In addition, the parahippocampal cortex and the posterior cingulate cortex are functionally connected, probably in a circuit devoted to memory and emotion.
41 In the perspective of a neural network of anxiety, the dysfunction of the posterior cingulate gyrus may contribute to some aspects of the cognitive profile observed in trait and clinical anxiety, such as perceptual biases in the interpretation of the environment, and perhaps also the negative memory bias observed in nonclinically anxious individuals.
A PET study has demonstrated an inverse correlation between resting dorsolateral PFC perfusion and state anxiety in older individuals, with a curvilinear relationship between asymmetric perfusion in the dorsolateral PFC and state anxiety.
43 Dorsolateral PFC has well known functional connections with amygdala, parahippocampal gyri, and posterior cingulate, a network of regions devoted to emotion processing.
40 Given the important contribution of dorsolateral PFC in selective attention,
44 we hypothesize that selective attentional biases observed in highly anxious individuals
45 may be associated with structural variability and reduced competence of this region of the prefrontal cortex.
We found that regions of the left ventral and lateral prefrontal cortex (Brodmann’s area 47) displayed positive correlation with STAI-S and STAI-T. Previous studies have demonstrated that these regions, functionally interconnected with the amygdala and inferotemporal regions, are typically activated by emotionally arousing stimuli
40 and are perhaps associated with externally generated emotional states.
46The reason for a positive correlation between gray matter volume in Brodmann’s area 44 and anxiety scores remains unclear. This finding may reflect the predisposition for the perseverative rumination observed in highly anxious individuals.
47Our findings support the hypothesis that nonclinical and pathological anxiety lie on a continuum, as they demonstrate analogous neuroanatomical features in addition to their known similar cognitive presentation. Decreased gray matter volume of brain structures involved in the expression of fear and anxiety circuit, including the parahippocampal-amygdalar region, cingulate, and PFC, may lead healthy individuals to have a more pronounced susceptibility to external stressors or physiologic events. The inverse correlation between regional gray matter volume and anxiety suggests that a larger number of neurons and a more extensive intra- and extraregional neuronal network are able to support more efficient functional capabilities, with ultimately lower levels of anxiety in subjects with larger gray matter volume in regions of the mesial temporal and prefrontal cortices. The cause of the mesial temporal and prefrontal gray matter volumetric variability seen in healthy individuals remains unknown and there are two possible explanations for it. Similarly to what is seen in patients affected by anxiety disorders, exposure to corticosteroids during stressful situations may cause modest structural gray matter changes in the amygdalar or hippocampal regions of participants without clinical anxiety disorders where corticoid receptors have been proved to be numerous; alternatively, temporo-mesial volume variability may preexist stressors due to genetic or developmental causes and predispose individuals to anxiety states.
30The literature suggests a correlation between anxiety and hippocampal volume; however in this study we did not find a correlation between anxiety scores and the volume of the hippocampal formation. This may have occurred for the following reasons: (1) possible suboptimal hippocampal segmentation using VBM given the absence of a sharp macroscopic limit between gray matter and white matter in this region, resulting in a lack of sensitivity to subtle volumetric changes of the hippocampus;
11,
28 (2) limitations due to our choice of anxiety scale; or (3) hippocampal volumetric changes may be specific to clinical anxiety. Massana et al.
29 reported lower bilateral amygdala volumes in patients affected by panic disorder than in comparison subjects. Nevertheless, in the present study, only the left amygdala volume displayed an inverse correlation with anxiety measures. The finding of a unilateral amygdala correlation may be consistent with observations of a different role in emotion processing for the left and right amygdala.
31The main strength of our work is the use of an automated volumetric technique to evaluate the association between brain morphometry and self-report scores from behavioral anxiety measures. Nevertheless our study has several limitations. First, we did not compare the results of the VBM analysis with other volumetric MRI techniques. VBM has the advantage of being a fully automated whole brain technique that detects regionally specific gray matter volume differences on a voxel by voxel basis, while commonly used manual or semiautomated volumetric MRI techniques are limited by being operator-dependent and subject to inter- and intraoperator variability. Therefore we believe that VBM is a more suitable tool for detecting subtle structural brain changes in healthy subjects than conventional volumetric MRI technique. Second, our study population included
young men and women. There is evidence that age and gender may represent a bias in the volumetric analysis of amygdala and hippocampus in humans. Total gray matter volume was included in the analysis as a confounding variable in order to eliminate global differences in gray matter that may have been due to factors such as age or gender, while allowing the evaluation of regional effects that were due to anxiety levels. Nevertheless, future investigations conducted on samples of healthy men and women separately may be helpful to confirm these findings or clarify gender differences. A recent report shows that there may be an inverse correlation in both genders between right hippocampal volume and higher anxiety-related personality trait, measured using the harm avoidance score from the Temperament and Character Inventory, whereas anterior prefrontal volume correlates with anxiety trait only in women.
18In the present study each participant underwent only one MRI study. In the future, gray matter volumetric changes and their relationship to anxiety ratings could also be evaluated longitudinally in healthy individuals. These longitudinal studies could help to establish whether persistently high levels of anxiety induce a gradual decrease of gray matter volume in certain regions. A longitudinal VBM analysis could reveal that certain healthy individuals who have relatively high levels of anxiety, but who do not meet the clinical criteria for anxiety disorders, may have consistently lower gray matter volume over time in certain brain regions than age-matched individuals with lower levels of anxiety. This analysis could provide further insights into the plasticity of the anatomical substrate of nonclinical and clinical anxiety.
In summary we found that in normal healthy individuals, regional gray matter volume of brain structures involved in the development of anxiety disorders are inversely correlated with self-reported anxiety measures. Our study suggests that volumetric variability of these brain regions may have an association with the development of an anxious personality trait. The causes of this structural variability, its exact relationship to function, and the temporal evolution of these volumetric findings remain unknown and are topics for future research studies.