V erbal memory impairments have been clearly established in patients with schizophrenia.
1 Major deficits in encoding processes have been noted, which suggest prefrontal cortex dysfunction.
2 It is important to determine the way and extent to which these impairments relate to clinical symptomatology. Memory efficiency is strongly related to functional outcome in this population,
3 and a better understanding of the aspects of memory deficits that are secondary to symptoms could guide the choice of cognitive and pharmacological therapies. However, this issue remains controversial. Although it has generally been shown that negative symptoms are associated with poor memory,
4 this association is weak and has not been observed in all studies.
Previous research from our group has suggested that depression, rather than negative symptomatology, is involved in the memory impairment.
5 This dimension is rarely taken into account in cognitive studies conducted in this population. However, depressive symptoms are prevalent in patients with schizophrenia, and they register as an important component in recent factor analyses of symptomatology in the disorder.
6 The commonly found association between negative symptoms and cognitive impairment may partly result from some overlap between depressive and negative symptomatology. Depression has been shown to affect verbal memory efficiency in a number of populations including children,
7 the elderly,
8 patients with affective disorders,
9 and patients with Parkinson’s disease.
10 In our previous schizophrenia sample, depression specifically affected effortful memory processes, in agreement with what was observed in individuals with primary depression.
11 Depression score was associated with impaired ability to organize lists of words in semantic categories, which reflects deep encoding. By contrast, it was not associated with the serial learning of items by rote rehearsal, which constitutes the most superficial way of encoding verbal information. The influence of depression on verbal memory efficiency in patients with schizophrenia has been reported by other groups.
12 –
14 However, several studies failed to reveal such a relationship.
15 –
18 Discrepancies may arise from differences in the scales used to assess depression, as well as from confounding factors, such as general verbal ability, or use of anticholinergic medication. Drugs with anticholinergic properties appear to affect verbal memory efficiency,
19 notably by impeding semantic organization of the material.
20With regards to negative symptoms, the numerous studies which have investigated their potential influence on memory impairment in schizophrenia patients have most often reported the correlations with a global negative symptom score. One reason for the weak associations observed may be that multiple symptoms are combined together, thereby obscuring possibly stronger relationships with specific symptoms. A more effective approach might be to investigate the role of individual symptoms. One symptom which is likely to be involved in poor cognitive performance is volitional deficiency. Our previous research indicated that, among all the negative symptoms assessed by both the Scale for the Assessment of Negative Symptoms (SANS) and the Positive And Negative Syndrome Scale for Schizophrenia (PANSS), only avolition from the SANS was significantly associated with verbal memory performance.
21 This might be a spurious result, stemming from a number of computed correlations. Alternatively, avolition might be a genuine factor in the impaired memory performance in this population. The correlation between verbal memory and SANS scores observed in several studies might have been mostly accounted for by this symptom, pointing to impairment in motivational processes. Successful performance relies not only on specific cognitive abilities, but also on the degree of invested effort to complete the task. Motivation could be seen as a cognitive energetic reserve to be drawn from for performing any task irrespective of its intrinsic difficulty. Recent studies have revealed the role of mental effort on cognition in schizophrenia.
22,
23 Schmand et al.
24 suggested that a motivational deficit underlies the association between negative symptoms and cognitive impairment in these patients. Although motivation might be crucial in cognitive and social functioning, its role has been understudied in schizophrenia, and warrants more systematic investigation. A better understanding of the impact of motivational processes would enable us to delineate more accurately the extent of the cognitive deficit. Besides, this would have practical implications for cognitive therapies if it were established that a significant amount of the observed impairment in memory and other cognitive domains is attributable to lack of motivation, rather than to impaired ability to perform the task.
Another clinical symptom that might be relevant for memory performance is attentional impairment. A disturbance in attention is commonly observed in patients with schizophrenia, and may contribute to their poor memory efficiency. Attention disorders might impede effective encoding by preventing the subjects from properly attending to the stimuli at the acquisition stage. Nevertheless, the few studies which have addressed the issue of the relationship between memory performance and various cognitive measures of attention in schizophrenia have not revealed a major role of attention deficit.
1 We observed in previous research that selective attention measured by the Stroop test was not associated with global verbal memory efficiency, or with any of the memory measures reflecting deep encoding.
25 However, deficits in selective attention were associated with impairment in the ability to encode and maintain series of either words or digits in memory by means of rote rehearsal. Likewise, distractibility, which stems from attentional dysfunction, has been suggested to interfere with the rehearsal processes of serial learning in schizophrenia patients
26 and children at risk for schizophrenia.
27 A specific relationship with serial encoding might similarly be observed when attentional impairments are assessed at the clinical level. Attention disorders are generally not considered a negative symptom, but they are nonetheless commonly evaluated as part of the SANS rating scale.
In this study, we wished to confirm in another schizophrenia sample our previous finding that depression has a significant effect on verbal memory efficiency, especially with regards to the deep encoding processes. A memory task yielding various levels of encoding was prepared. The most superficial encoding level was assessed by the forward digit span test, which merely requires learning sequences of digits. Three lists of words with increasing difficulty were administered. One list was not organizable; one was semantically organizable and contained typical instances of the semantic categories; the last list was semantically organizable with atypical examples, and presumably required even deeper processing than the typical one. We conducted regression analyses on the various memory scores to test the hypothesis that depression, rather than a global negative symptom score, was a significant predictor of verbal memory efficiency. More specifically, we expected the association with the recall of the organizable lists to be stronger than that with the recall of the nonorganizable list, and an even stronger correlation was expected for the atypical than for the typical organizable list. By contrast, no association between depression and the forward digit span was expected, since depression usually spares the superficial memory processes, and was unrelated to the efficiency of serial encoding in our previous study. Verbal IQ and use of antipsychotic medication with intrinsic anticholinergic activity were included in the regression analyses, in order to control for their potentially confounding effect.
Our second purpose was to test the hypothesis that certain clinical symptoms, commonly assessed in schizophrenia but not studied on their own, have a significant impact on memory performance. The SANS was used for clinical symptom assessment, and an extensive score for each symptom was tallied by summing the subscores which constitute the symptom. On the basis of our previous results, we hypothesized that avolition score would be significantly correlated with the total number of recalled words assessing global verbal memory efficiency. This would confirm an association which was not expected in our previous study, but rather emerged from a post-hoc analysis. Possible correlations between avolition score and other memory measures were explored, to determine the scope of the effect. Attention disorders were expected to be significantly associated with serial learning assessed by the forward digit span, in agreement with the findings from previous studies. By contrast, they were not expected to be associated with the other memory scores, which reflect deeper processes than serial learning. No significant association between the other SANS symptoms (affective flattening, alogia, and anhedonia) and any of the memory measures was expected.
METHODS
Participants
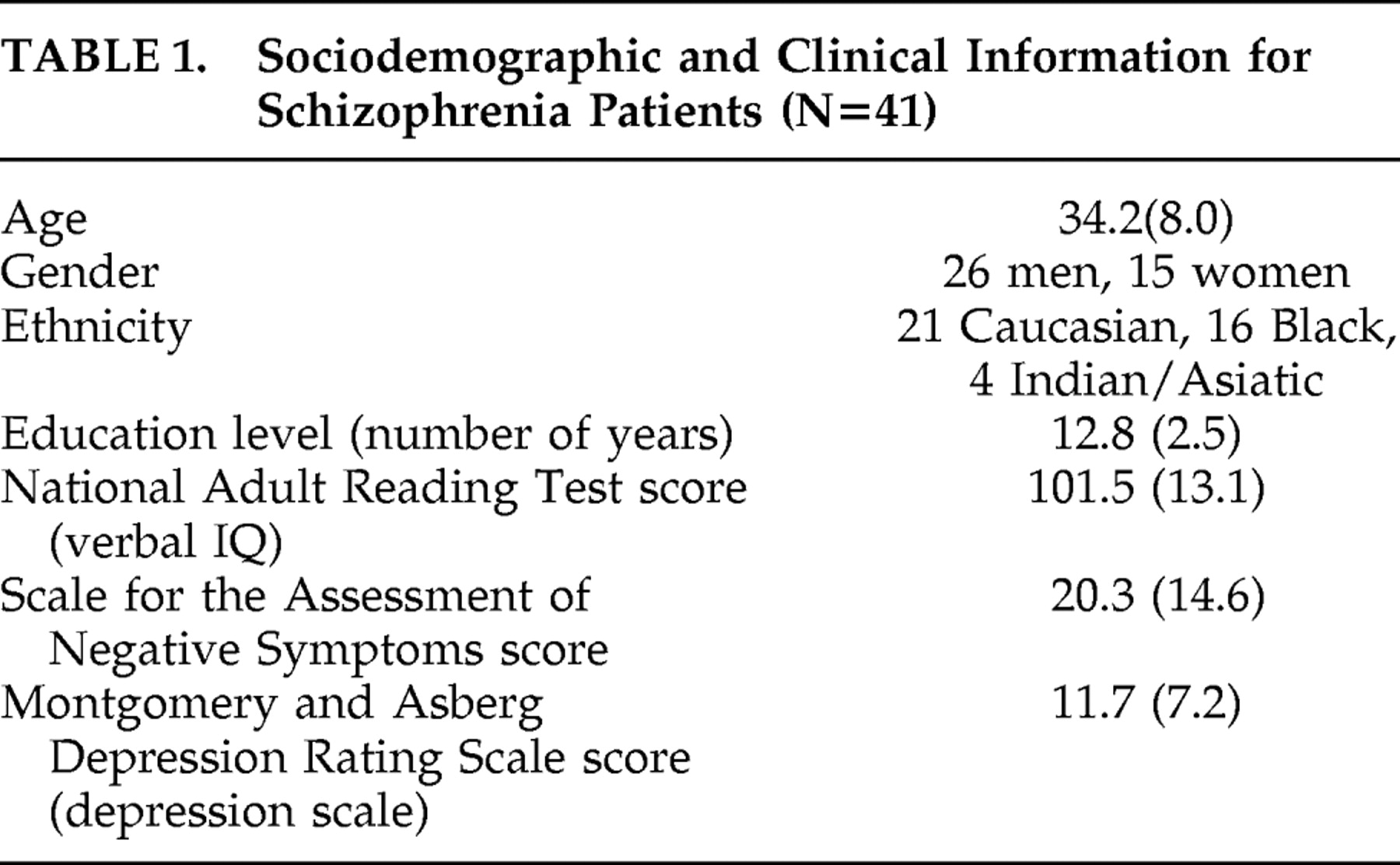
Forty-one patients with schizophrenia (8 inpatients) were recruited at the Maudsley Hospital, London (see
Table 1 for sociodemographic and clinical information). They were all taking antipsychotic medication except three patients who were declining to take any medication. Fourteen of the patients were taking antipsychotic medication with intrinsic anticholinergic activity (olanzapine, clozapine, zuclopenthixol). Diagnosis was made on the basis of DSM-IV criteria by two senior psychiatrists who used patients’ history and chart review, and reached a consensus. Patients with substance abuse and comorbid medical or psychiatric conditions were excluded. Ethical approval for the study was obtained from the South London and Maudsley NHS Trust Ethics Committee. After a full explanation of the study, subjects provided written informed consent to participate.
Clinical Ratings
The Montgomery and Asberg Depression Rating Scale (MADRS
28 ) was used to assess severity of depression. It includes 10 items (apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, suicidal thoughts), with a possible score ranging from 0 to 6 for each item. The total score range in the sample was 0–26. Negative symptoms and attention disorders were assessed with the SANS (score range=0–62). A score for each symptom was computed by summing the subscores which constitute this symptom (affective flattening: range=0–21, mean=6.2, SD=6.5; alogia: range=0–12, mean=2.3, SD=3.1; avolition/apathy: range=0–12, mean=3.9, SD=2.9; anhedonia: range=0–14, mean=6.8, SD=4.5; and attention disorders: range=0–7, mean=1.1, SD=1.7). The psychiatrists were blind to cognitive results during clinical assessment. Likewise, the experimenter (GB) was blind to patients’ symptoms during testing.
Forward Digit Span
The forward digit span test from the WAIS-Revised (WAIS-R
29 ) was administered. Increasingly long series of digits had to be repeated in the same order. The measure used was the total number of correct trials. This task was assumed to assess the superficial memory processes of serial encoding.
Lists of Words
Eight lists of 16 concrete words, equivalent in average number of syllables, were prepared. Four of the lists were made up of non semantically-related words, and only one of them chosen at random was presented for immediate recall. Four other lists were organizable into four semantic categories selected from Battig and Montague.
30 Two of them were made up of four typical instances within each category, whereas the other two were made up of four atypical instances. The words were distributed randomly within the lists, so that the semantic organization was not obvious. The subjects were presented with one typical and one atypical semantically organizable list. The choice of the target lists, as well as the order of presentation of the semantically organizable lists, was counterbalanced among subjects.
All the lists of words were printed. The nonorganizable list was administered first. The subjects were presented with the list and informed that they had 45 seconds to learn the words, and had to read them aloud at least once. Immediately after learning, they were provided with a blank sheet and required to write down all the words they could remember in any order. No time limit was imposed for free recall. Then one of the organizable lists was administered following the same procedure, and finally the other organizable list. The subjects were not informed that certain lists were semantically organizable. The numbers of words recalled in each list were tallied and used as dependent variables. In addition, a global verbal memory measure was computed by summing the number of words recalled in the three lists (total number of recalled words).
All the memory and clinical variables followed a normal distribution except the attention disorder score from the SANS, which was normalized by square-root transformation before data analysis. All statistical tests were two-tailed.
RESULTS
Memory Scores
The scores obtained by the patients on the memory measures were as follows: forward digit span: mean=7.0, SD=1.8; number of recalled words: nonorganizable list: mean=5.5, SD=2.1; typical organizable list: mean=6.4, SD=3.0; atypical organizable list: mean=5.0, SD=2.7; total number of recalled words: mean=16.9, SD=6.9. An analysis of variance (ANOVA) conducted on the number of words recalled in each of the three lists revealed a significant main effect of the type of list (F=7.54, df=2, 80, p<0.001). Notably, fewer words were recalled in the atypical than in the typical organizable list (F=15.1, df=1, 40, p<0.0001). The role of list structure on recall efficiency in mostly the same sample, as well as the impairment relative to a healthy control group, was fully discussed in a previous paper.
31 The patients were highly significantly impaired on the forward digit span and on the number of recalled words.
Associations of Memory Scores With Negative and Depressive Symptomatology
A matrix of the bivariate correlations among the memory measures and SANS, MADRS, and National Adult Reading Test (NART) scores is presented in
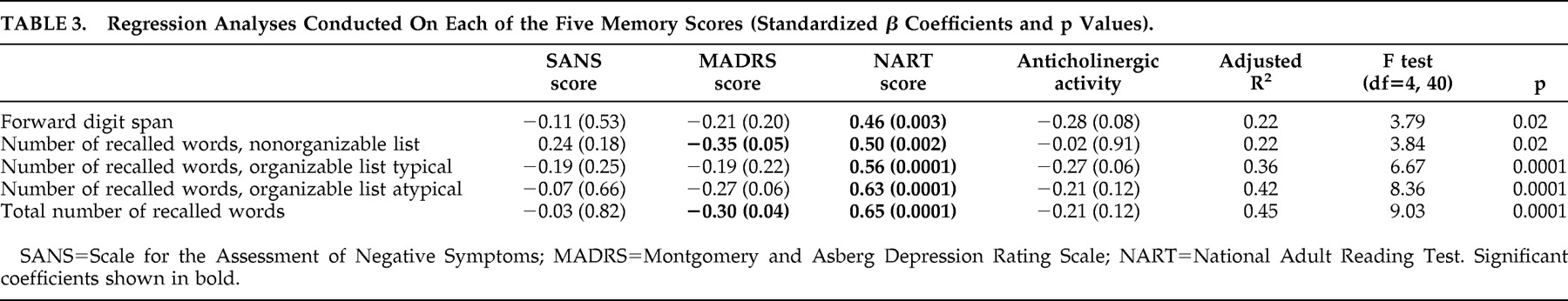
Table 2 . The MADRS and SANS scores were significantly intercorrelated (r=0.52, p<0.001), which demonstrates the necessity to include these two variables together in regression analyses, so as to study the independent contribution of each to the memory impairment. Regression analyses were conducted on the memory measures. The SANS and MADRS scores were entered as predictors. The NART score, reflecting verbal intellectual level, and the use of antipsychotic medication with intrinsic anticholinergic activity (categorical variable) were entered as well, in order to control for their potentially confounding effect. All four predictors were entered together in the regression. Results are presented in
Table 3 .
The NART score was a highly significant predictor of all the memory measures. The SANS score was not a significant negative predictor of any of the memory measures. If anything, it presented a positive, although nonsignificant, association with the number of words recalled in the nonorganizable list. By contrast, the MADRS score was a significant negative predictor of the total number of recalled words, as expected. Contrary to our hypothesis however, depression was not significantly associated with the recall of the typical organizable list, and was only marginally significantly associated with the recall of the atypical organizable list. The association between MADRS score and forward digit span was far from significant.
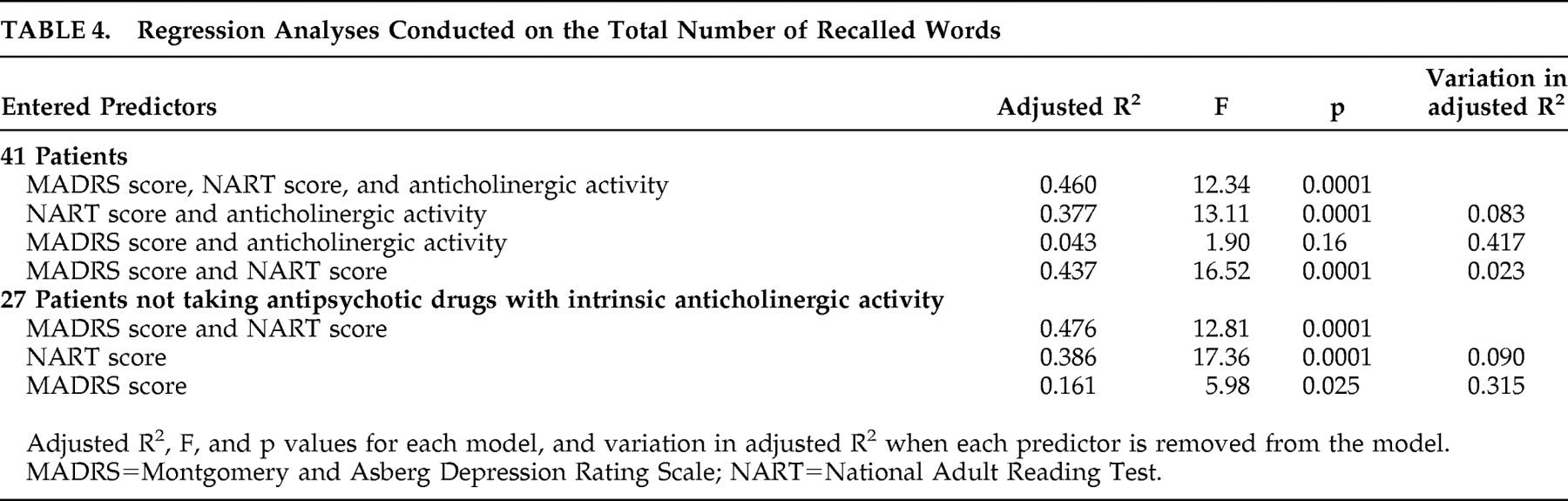
The type of antipsychotic medication made a marginally significant contribution to the recall of the typical organizable list. It should be noted that this contribution became significant when the scores from the typical and atypical organizable lists were summed (β=−0.26, p<0.05). This finding indicates that the patients who took antipsychotic treatment with intrinsic anticholinergic activity were impaired in the recall of these lists, relative to the other patients. In view of the significant role of antipsychotic medication, we recomputed a regression analysis in the subsample of 27 patients who did not take any antipsychotic drug with anticholinergic activity, after checking the normality of the score distributions. The criterion variable was the total number of recalled words. The SANS, MADRS and NART scores were entered together as predictors. The model yielded an adjusted R 2 =0.47 (F=8.62, df=3, 26, p<0.001). Results show that the SANS score did not make any negative contribution to the total recall score (β=0.15, p>0.43), whereas the strength of the association with the MADRS score was enhanced relative to that observed in the whole sample (β=−0.42, p<0.04). The NART score remained a highly significant predictor (β=0.60, p<0.0001).
In order to quantify the proportion of memory variance that was explained by depression, NART score, and drugs with intrinsic anticholinergic activity, we examined the variation in adjusted R
2 induced by removing each of these factors from the model. A regression analysis on the total number of recalled words was conducted in the whole sample, using MADRS score, NART score, and type of antipsychotic medication as predictors entered all together. The SANS score was omitted, since it did not show any effect on the total recall score. Then each predictor was alternately removed from the regression analysis, and the resulting variation in adjusted R
2 was studied. The results are reported in
Table 4 . It appears that the MADRS score explained 8.3% of the variance in total recall, while the NART score explained 41.7%, and the use of antipsychotic medication with intrinsic anticholinergic activity explained 2.3%. Analyses conducted in the subsample of 27 patients who did not take antipsychotic treatment with intrinsic anticholinergic activity revealed that 9% of the variance in total recall was accounted for by the depression score, and 31.5% by the NART score.
Complementary analyses with the same predictors were conducted on the scores obtained on the nonorganizable versus semantically organizable lists, to test the hypothesis that depression especially affects the learning of the organizable lists, requiring deep encoding processes. In the whole sample, the MADRS score accounted for 3.5% of the variance in the recall of the nonorganizable list, and for 8.2% of the variance in the summed recall of the two semantically organizable lists. In the subsample of 27 patients, the proportion of variance explained by depression was 0.1% for the nonorganizable list, and 10.9% for the summed two semantically organizable lists.
Correlations Between Memory Scores and Individual SANS Symptoms
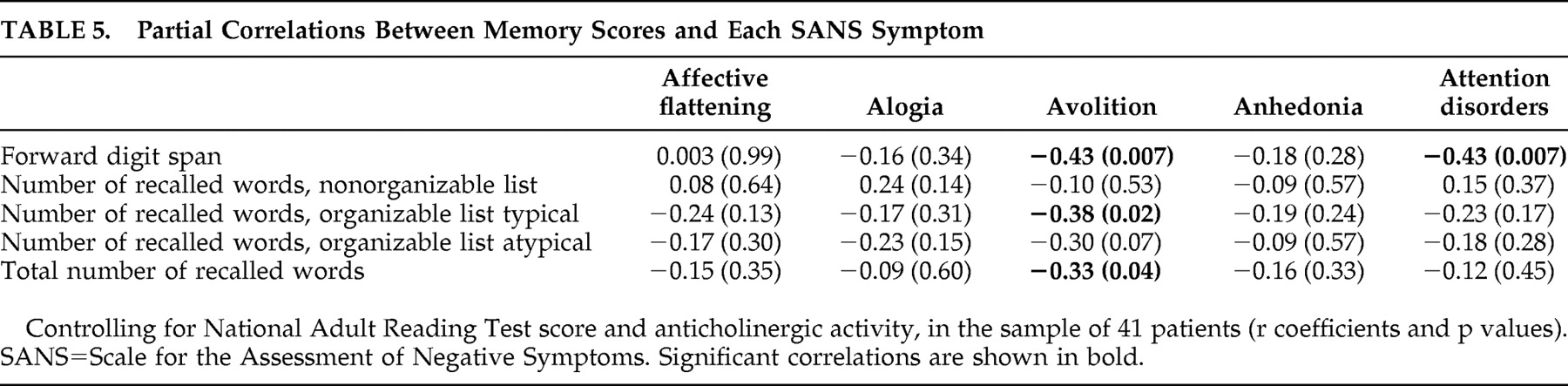
We then tested the hypothesis that, in spite of the lack of association with the global negative symptom score, avolition and attention disorders were associated with certain memory measures. The correlations between the memory measures and each of the SANS symptom scores were computed, after controlling for NART score and use of antipsychotic medication with anticholinergic activity. Results are presented in
Table 5 .
The avolition score was significantly correlated with the total number of recalled words, as expected. It was also significantly correlated with the forward digit span and with the recall of the typical organizable list, and tended to be correlated with the recall of the atypical list. In agreement with our hypothesis, attention disorders were significantly correlated with the forward digit span, but not significantly related to any word recall score. No significant or trend level correlation emerged between the other SANS symptoms (affective flattening, alogia, and anhedonia) and any of the memory scores.
The patients who did not present attention disorders might obscure any potential association with memory. We therefore recomputed the partial correlations between attention disorder score and memory measures in the subsample of 18 patients with attention disorders (score≥1), after checking that all studied variables followed a normal distribution. The negative correlation between attention disorder score and digit span remained significant (r=−0.54, p<0.03). The negative correlations with the other memory scores were far from significant (p>0.31 in all cases).
DISCUSSION
Memory efficiency has been shown to play a crucial role in daily living and social outcome in patients with schizophrenia.
3 It is therefore particularly important to determine the factors that are involved in the pronounced verbal memory impairment consistently observed in this population, in order to develop more targeted and effective treatment. Our previous finding concerning the role of depression was an important one, which called for replication.
5 In the current study, regression analyses involving both negative and depressive symptomatology indicated that the global negative symptom score did not present any significant association with any memory measure. By contrast, the depression score was significantly associated with the total number of recalled words, while it was unrelated to the forward digit span reflecting superficial encoding by serial learning. Our results therefore confirm that depression, rather than negative symptoms, contributes to the verbal memory impairment in patients with schizophrenia, at least insofar as deeper mechanisms than serial learning are involved. A similar pattern of associations between depressive symptoms and verbal memory measures requiring deep, but not superficial, encoding was reported by other groups.
12,
14It should be noted that the association with depression, although significant, was moderate. Correlational and regression analyses in the whole sample suggested that this association did not concern the semantically organizable lists of words any more than the nonorganizable list, contrary to our hypothesis. However, the relationship between memory efficiency and depression appears to be influenced by the use of antipsychotic drugs with intrinsic anticholinergic activity. The association between MADRS score and total recall was enhanced in the subsample of patients who did not take this type of medication (β=−0.42 in the subsample versus β=−0.30 in the whole sample). Furthermore, in this subsample, depression explained a substantial proportion of the variance in the recall of the semantically organizable lists (10.9%), but not in the recall of the nonorganisable list (0.1%). This suggests that depression affects the deep processes of semantic encoding.
Overall, the effect of depression appears less striking than that observed in our previous sample.
5 The reduced magnitude of this effect is not likely to be due to the task, which was at least as demanding as that designed in the previous study. The 21-item Hamilton Depression Rating Scale used in the previous study probably captures more of the relevant features of depression than the 10-items MADRS. Depression includes a variety of components, some of them being also part of negative symptomatology (e.g., blunted affect, social avoidance, disturbance of volition, psychomotor retardation). Our pattern of data suggests that depression affects memory through its volitional component. Other aspects more specific to the depressive state also play a role. More particularly, anxiety, which is included in the Hamilton Depression Rating Scale, was strongly associated with impaired ability to use the semantic organization in our previous data. It accounted for a great part of the association between depression score and memory efficiency. The fact that anxiety is not included in the MADRS probably explains the weak association with the recall of the organizable lists in the current data. Fatigue might also contribute to the association between memory efficiency and depression. Future studies of memory functioning in patients with schizophrenia should take into consideration the potentially deleterious role of depression. The association of memory performance with depressive severity, which has been robustly established in patients with primary depression,
9,
11 and also observed in other clinical
10 and nonclinical
7,
8 populations, is underestimated in schizophrenia research. Assessment of depression and anxiety should be routinely included in cognitive studies of schizophrenia, which have so far most often focused on positive and negative symptomatology. Clinicians should also consider the role of depressive symptoms when they attempt to remediate cognitive impairment by either behavioral or pharmacological therapies.
The lack of significant associations between SANS score and any of the memory measures in the regression analyses is consistent with a meta-analysis indicating that negative symptomatology only accounts for a small amount of the memory deficit.
4 The finding of significant associations in some studies might have been influenced by confounding factors, notably verbal IQ, which appears to have a strong impact on all the memory measures. Our results underscore the importance of controlling intelligence level when studying the relationships between cognition and clinical symptoms. Furthermore, the reported associations of verbal memory with negative symptoms in the literature might partly arise from overlap between negative and depressive symptomatology. The matrix of bivariate correlations in our study revealed significant or trend associations between SANS score and word recall. However, these associations disappeared when the data were submitted to regression analyses, which controlled for the effect of the other factors. This observation suggests that these bivariate correlations were either accounted for by an element common to negative and depressive symptomatology, or an artifact of the strong associations between memory and verbal IQ.
As expected, avolition was the only symptom from the SANS to be significantly correlated with the total number of recalled words. This finding illustrates the advantage of studying the influence of individual symptoms, rather than using a global negative symptom score. The size of the correlation with avolition was modest, yet similar to that observed in our previous study (r=−0.33 in this sample versus r=−0.36 in Brébion et al.).
21 In the current data, avolition was also associated with other memory scores, which suggests the pervasiveness of its negative effect on memory. The associations between memory efficiency and SANS score observed in several studies might have been triggered by the avolition item. Our findings corroborate recent reports demonstrating the role of mental effort on cognitive efficiency in schizophrenia,
22,
23 and give support to Schmand et al’s
24 suggestion that the effect of negative symptoms on cognitive efficiency stems from motivational deficit. Regardless of task difficulty, one cause of patients’ poor memory performance might be failure to invest sufficient mental effort to perform the task. Patients’ cognitive deficit may be overestimated due to their lack of motivation to work at the maximum of their capacities. The issue of motivation, as critical as it might be, has been poorly investigated so far, prompting the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) committee to call for more research on this topic.
32 The motivational processes could be assessed with extensive rating scales, as well as with behavioral tests. If a significant impact of motivation on memory, and possibly other functions, were robustly established in this population, this would raise hope that patients would benefit from cognitive rehabilitation techniques that would aim at improving their motivation to perform the task.
With regards to clinically rated attention disorders as defined in the SANS, their influence on memory efficiency in this sample was very limited. Attention disorder scores were not associated with global verbal recall, or recall of either type of word list. This corroborates the few studies which assessed attention by means of cognitive tasks and did not reveal a major role on memory efficiency.
1 However, attention disorders were significantly associated with impairment in encoding series of digits. This specific association confirms that obtained in our previous study, in which attention disorders were assessed at a cognitive level by means of the Stroop test.
25 It also corroborates findings from other groups.
26,
27 Attention deficit, assessed by either cognitive measure or clinical rating, seems to affect verbal memory only insofar as it disrupts the serial learning processes. Admittedly, the attention score had a narrow range, thereby reducing the chance of detecting significant associations. In addition, the SANS rating subscale captures only restricted aspects of attention disorders, which limits the scope of the conclusions. Considering that attention is a complex construct, various clinical and behavioral measures of attention should be used to confirm that impairment in this function does not broadly affect verbal memory efficiency in patients with schizophrenia, but merely interferes with serial encoding.
A limitation of this study is the relatively small size of the sample. The regression analyses may have been underpowered, and a stronger effect of depression might have emerged in a larger sample. Furthermore, the MADRS was not designed to assess depression in schizophrenia patients. Future studies should use other scales more specific to this population, such as the Calgary Depression Scale. Lastly, the finding of associations between clinical symptoms and memory measures does not necessarily mean that these symptoms influence memory efficiency. The dorsolateral prefrontal cortex has been shown to play a role in both symptoms of avolition and depression.
33 This brain area has also been involved in the long-term memory deficits in patients with schizophrenia.
34 It is possible that the underlying neurobiological abnormalities that give rise to impaired memory functioning in this disorder also concomitantly give rise to the clinical manifestations of depression and avolition. Nevertheless, our findings suggest that more consideration should be given to these two clinical symptoms in experimental investigations of verbal memory and other cognitive functions in schizophrenia, as well as in therapeutic interventions aiming at improving cognition.
Acknowledgments
Dr. Brébion was funded by grants from the Leverhulme Trust and from the Bial Foundation, and by the Alexander Gralnick Grant for Research on Schizophrenia, American Psychological Foundation. We gratefully acknowledge awards from Ian Karten Charitable Trust and Remedi.