R epetition-induced reduction in P50 amplitude (P50 gating) could reflect a preattentive mechanism that filters out irrelevant information, affecting cognitive and motor processes and task performance.
1,
2 Strong evidence exists that P50 gating is impaired in psychosis-related disorders, such as schizophrenia,
3 and anxiety/stress-related disorders, such as posttraumatic stress disorder.
4 Although not addressed directly, recent findings also suggest weaker gating in disorders associated with pathological impulsivity, including alcohol abuse,
5– 7 substance abuse,
5,
8,
9 adult attention-deficit hyperactivity disorder (ADHD),
10 bipolar disorder without psychosis,
11 and violence in schizophrenia,
1 even though in most of these studies results may be affected by overlap with psychosis- or anxiety-related disorders. Interestingly, Thoma et al.
7 showed that subjects with combined alcohol abuse disorder and schizophrenia had more severely impaired P50 gating than either disorder alone, suggesting partially independent mechanisms.
Antisocial personality disorder (ASPD) is a disorder of pathological impulsivity without psychosis or anxiety. True ASPD, with childhood onset of antisocial behavior, differs in clinical characteristics from adolescent- or adult-onset antisocial behavior (AAB) by being associated with more pervasive sociodevelopmental, cognitive, and behavioral disturbances.
12 In this pilot study we investigated the relationship between P50 gating and pathological impulsivity directly in ASPD and AAB patients without past or current psychosis- or anxiety-related disorders. We expected impaired P50 gating in subjects with antisocial behavior relative to healthy comparison subjects.
METHODS
Participants
All subjects were recruited from the general population through advertisements. Participants completed the Structured Clinical Interview for DSM-IV axis I and axis II disorders (SCID-I and SCID-II).
13 Participants with antisocial behavior met criteria for ASPD or AAB. Healthy comparison subjects were required to have no current or past axis I or axis II disorders and to have no first-degree relatives with axis I or II disorders. Further criteria were age between 18 and 55 years old, hearing at least 60 dB, (corrected-to-) normal vision, no head trauma or epilepsy, and no current use of psychoactive medicine. Participants signed informed consent before participation. The study complied with the Declaration of Helsinki and was approved by the Committee for the Protection of Human Subjects institutional review board for the University of Texas Health Science Center at Houston.
There were sixteen antisocial patients (mean age=34.50 years, SD=9.16) of whom three were female; the group included nine patients with ASPD and seven with AAB. Antisocial personality disorder patients had two to 12 conduct disorder symptoms. Five AAB patients had one symptom for conduct disorder, including skipping school, shoplifting, lying, fighting, and intentionally inflicting pain on people. Across both groups, patients had three to six adult antisocial symptoms. Coexisting diagnoses included current/past alcohol/substance abuse/dependence (n=10), passive aggressive personality disorder (n=1), and narcissistic personality disorder (n=2). Seven participants had no comorbid disorders. The subjects had histories of imprisonment or probation for nonviolent offenses (n=6), theft (n=6), or assault (n=2); data were missing from two patients. The two groups did not differ in age, highest level of education, gender or ethnic distribution, axis I or II disorders, current/past alcohol/substance abuse/dependence, criminal history, and in the number of symptoms for adulthood antisocial behavior. Participants were not excluded other than based on the above-mentioned criteria. Fifteen age-, gender-, and ethnicity-matched subjects (mean age=32.0, SD=9.64, six female) were selected from a larger database to serve as healthy comparison subjects. Subjects were selected if they had no first-degree relatives with any psychiatric disorder, drug abuse, or alcohol abuse, and if they had no symptoms for conduct disorder.
Procedure
Participants had to have negative screens for drugs (RediCup®, Redwood biotech, Santa Rosa) and alcohol (Alco-Sensor III, Intoximeters Inc., St. Louis), and could not consume caffeine for 8 hours, or smoke for 1 hour, before testing. Participants performed two blocks (80 pairs of clicks) of the paired-click paradigm. Paired stimuli, designated S1 and S2 (intrapair interval: 500 msec, interpair interval: 8–10 msec), were clicks (40 msec, 80 dB, 1000 Hz, 4 msec fall-rise) presented binaurally through headphones using STIM software (Neuroscan, Inc., El Paso). Participants were instructed to listen passively to the clicks, relaxing and sitting quietly with their eyes open and fixated.
Data Acquisition and Analysis
Trait impulsivity was assessed with the Barratt Impulsiveness Scale (BIS-11), measuring three subfactors: nonplanning (planning, self-control), motor (acting without thinking, perseverance), and attentional impulsivity (sustained attention, concentration).
14 Established procedures were followed for P50 assessment.
15 An EEG (sample-rate: 1000 Hz, filter: 0.1 to 100 Hz, amplifier: 10,000x through SynAmps) was recorded with the Acquire module of SCAN 4.3 software (NeuroScan, El Paso) from 32 electrodes attached in a Quik-cap (Neuromedics Neuroscan, El Paso). Signals were referenced to both mastoids. The ground electrode was attached anteriorly of F3-F4 extending to the midline. Electrooculograms were assessed with electrodes above and below the right eye and both outer canthi. Impedances were kept below 5 kΩ.
Off-line, using the edit module of SCAN 4.3 software, raw signals were filtered between 1 and 50 Hz (48 dB/oct roll-off, zero-phase shift). Eye blinks were detected and corrected semiautomatically. The data were epoched between −100 msec to 400 msec relative to stimulus onset, and baseline corrected. Trials with artifacts were rejected, together with the trial of the paired stimulus. Sixty-four to 80 pairs were retained. Before averaging the data for S1 and S2 separately, the data were filtered at 10 Hz (high-pass), optimizing scoring of the P50. The P50 was assessed at Cz as the most positive peak between 35 and 85 msec, scored by investigators (NNB and SB) unaware of subject diagnosis. The amplitude was scored relative to the amplitude of the preceding negative trough. Scalp distributions were taken into account if identification was ambiguous, in which peaks were selected with a frontocentral distribution. For S2 the P50 had to peak within 10 msec of the P50 for S1, unless components fell outside the windows (maximum 15 msec) as revealed by iso-potential maps.
Statistical Analysis
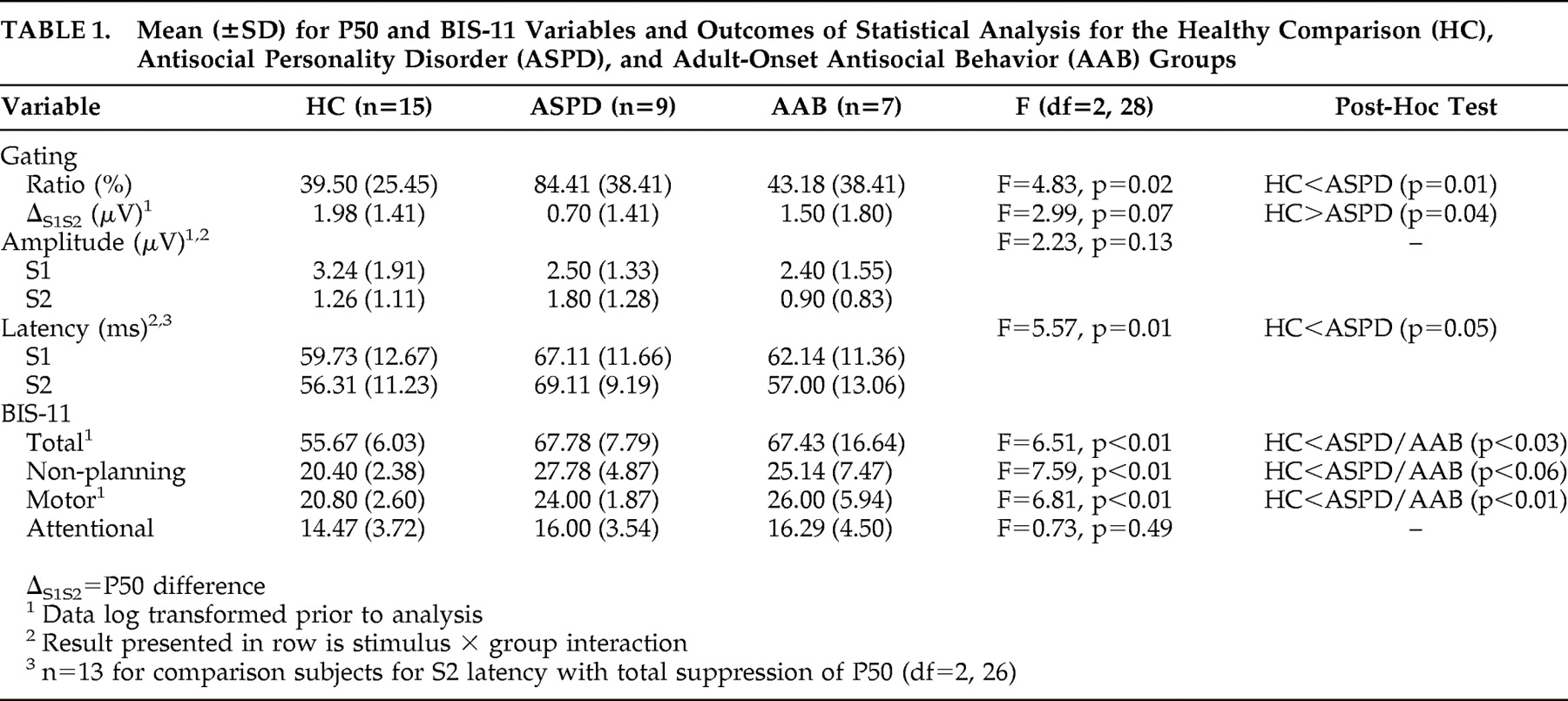
P50 amplitude, latency, ratio ([S2 amplitude /S1 amplitude ] × 100), and difference (S1 amplitude −S2 amplitude ) were assessed. A higher ratio and lower difference reflect impaired gating. Group differences were tested with univariate (BIS-11, ratio, difference) and repeated measures analysis of variance (ANOVA) (amplitude, latency) (Stimulus, S1, S2, as within-subjects factor) with group (healthy comparison, ASPD, AAB) as between-subjects factors. Significant group effects (p<0.05, two-tailed) were tested further with Dunnett’s post-hoc test in which the two experimental groups were compared with the comparison group.
DISCUSSION
Patients with antisocial personality disorder (ASPD) had impaired P50 gating and delayed peak latencies, consistent with abnormal preattentional filtering of information in pathological impulsivity and with reports of weaker gating in subjects with other disorders related to pathological impulsivity.
5 –
11 Additionally, the association between P50 ratio and number of symptoms for conduct disorder, but not adult antisocial behavior, suggests a continuum of weaker P50 gating from AAB to ASPD depending on the severity of antisocial behavior in childhood.
Participants in the comparison and clinical groups reacted to advertisements in the local press, suggesting both groups are a reflection of the general population. Healthy comparison subjects had no first-degree relatives with a psychiatric disorder and no symptoms of conduct disorder, whereas the clinical subjects had histories of extreme impulsivity-related behaviors. Our results may therefore only generalize to extreme groups.
Subjects in our samples had no history of psychosis- or anxiety-related disorders, so our findings suggest that besides being associated with psychosis- and anxiety-related disorders,
3,
4 impaired P50 gating could additionally be a marker for impulsivity-related disorders. Recent research suggested an association between P50 gating and frontal lobe activity.
16 Difference in frontal lobe functioning may explain weaker P50 sensory gating across the different psychiatric disorders, including ASPD.
17 The limitations of this study are the limited number of subjects and high incidence of substance abuse. Substance abuse or withdrawal could have confounded our findings,
5,
9 although the AAB and ASPD samples had similarly increased prevalence of substance or alcohol-related disorders. This aspect requires more research.
Barratt Impulsiveness Scale (BIS-11) scores were similar in subjects with ASPD and AAB. These outcomes are consistent with results found by Moffitt and Caspi
12 that child- and adolescent-onset antisocial behavior were both associated with delinquency, impulsivity, and substance abuse, but that childhood-onset subjects had more pervasive developmental, cognitive, and social disturbances. On the other hand, although impulsivity is an important aspect of ASPD, it does not explain the entire clinical presentation of the disorder.
18 The similarity in BIS-11 scores could be due to the BIS-11 relying on personal insight and the ability to estimate an average level of behavior across a certain time period. Although no information exists on what time period people take into account when filling out the BIS-11, it could be that this time period does not reach back into childhood and adolescence. As ASPD and AAB subjects do not differ in demographics and impulsive behaviors in adulthood, the BIS-11 could be a reflection of behavior during part of adulthood. The P50, on the other hand, could reflect a genetic predisposition for psychiatric disorders,
19 including early-onset antisocial behavior. This would be similar to findings for the P300 component: a less pronounced P300 was associated with earlier onset of antisocial behavior in subjects abusing drugs, and was correlated with more conduct disorder symptoms.
20 These findings suggest that ASPD is associated with biological correlates, whereas AAB could be associated more with environmental correlates. Impaired gating may therefore be related to more specific aspects of impulsivity or development that distinguish early-onset ASPD from adult antisocial behavior.
Acknowledgments
This study was supported in part by the Pat R. Rutherford, Jr., Chair in Psychiatry (ACS) and by National Institute of Health grants RO1-MH 69944 (ACS), RO1-DA08425 (FGM), KO2-DA00403 (FGM), RO1-MH58784 (NNB), and UL1-RR024148 (CTSA) (General Clinical Research Center, University of Texas, Houston).